The Use of Hcv Nat+ Organs in Hcv Negative Recipients with On-site Specialty Pharmacy Services: A Win for the Patient, the Transplant Center, and Society?
M. Person, N. Patel, W. Simerlein, H. Meadows, D. DuBay, D. Taber
Medical University of South Carolina, Charleston, SC
Meeting: 2021 American Transplant Congress
Abstract number: 384
Keywords: Hepatitis C, Pharmacoeconomics, Viral therapy
Topic: Clinical Science » Ethics » Non-Organ Specific: Economics & Ethics
Session Information
Session Name: A Penny for Your Thoughts: the Economics and Psychosocial Aspects of Transplant
Session Type: Rapid Fire Oral Abstract
Date: Tuesday, June 8, 2021
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:10pm-6:15pm
Presentation Time: 6:10pm-6:15pm
Location: Virtual
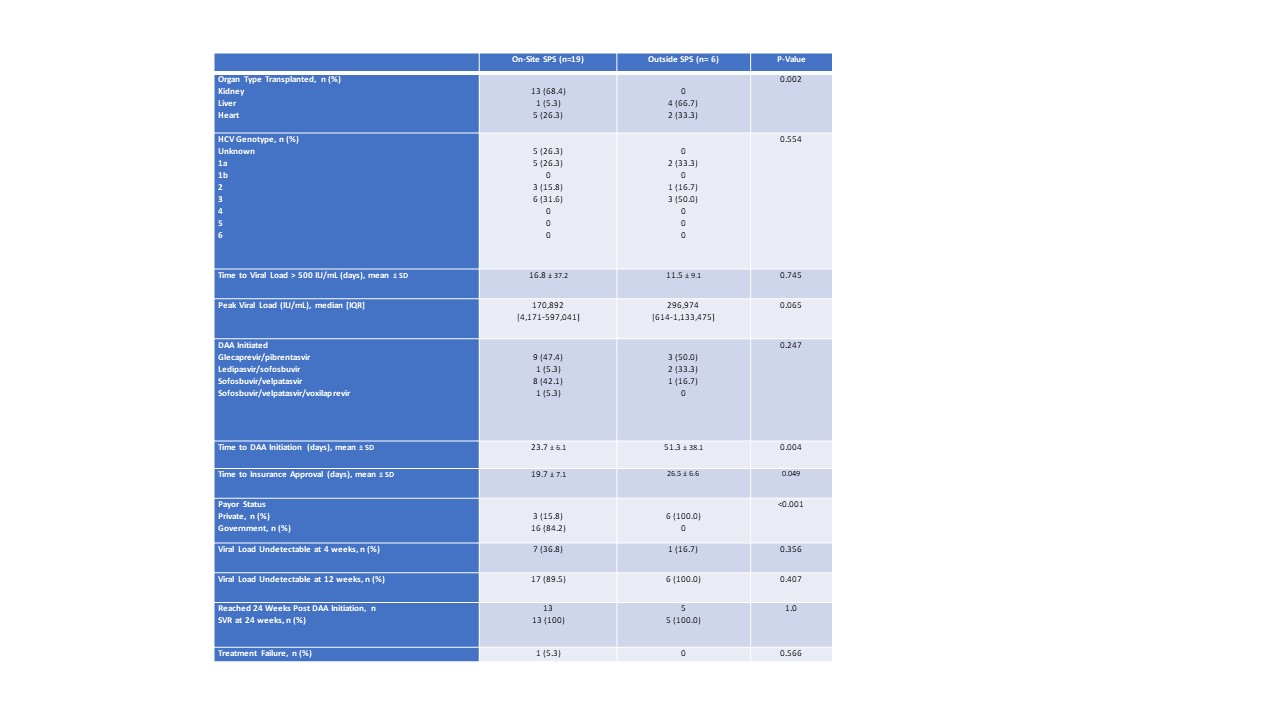
*Purpose: Use of HCV AB positive organs (HCV AB+) with active viremia (NAT+) relies on appropriate and timely use of direct acting antiviral therapies (DAA). These are cost restrictive agents and thus, patient access to DAAs may be improved with the use of specialty pharmacy services (SPS). We hypothesized that on-site SPS improve patient access and adherence by facilitating the completion of prior authorizations and patient assistance while ensuring medication access and generating revenue for the institution.
*Methods: This was a single center retrospective analysis performed from 1/1/2019-11/1/2020. Included patients are those who had documented HCV labs 12 weeks from the day of DAA initiation. For display of results, patients were divided by location of fill, either on-site SPS or an outside SPS. Primary outcomes were time to insurance approval and start of therapy, rate of adherence, patient out-of-pocket cost for 12 weeks of therapy, and revenue generated per patient.
*Results: Sixty-four patients were included, 40 NAT+, and of those 25 had documented HCV labs at 12 weeks from DAA initiation and are included in these results. Of the patients that used on-site SPS, 25 (100%) required at least 1 prior authorization and 6 (24%) required an appeal for insurance approval. Use of the on-site SPS reduced the time to gain insurance approval by one-third (27 vs 20 days, p=0.049) and time to start DAA by roughly half, from 51 to 24 days (p=0.004). On average, patients that filled at on-site SPS had a total out-of-pocket cost of $10 for 12 weeks of DAA therapy and had 99.8% adherence to therapy. DAA therapy generated $38,964.45 of net revenue per patient for the institution per each 12 week treatment course. Kidney recipients that received an HCV AB+ organ waited 2.78 years on the transplant list, as compared to the average wait time at our institution of 4.35 (p<0.01) during a similar time period. One patient died during this time frame, though this patient received an HCV AB+ NAT- organ and did not require DAA therapy.
*Conclusions: Utilizing HCV AB+ organs results in significantly shorter duration of time spent on the transplant waitlist. When NAT + organs are utilized, on-site SPS can facilitate a significantly shorter time to DAA insurance approval and initiation of therapy, while achieving very high adherence rates and generating a significant net revenue option for the institution. This represents the ideal win-win-win scenario.
To cite this abstract in AMA style:
Person M, Patel N, Simerlein W, Meadows H, DuBay D, Taber D. The Use of Hcv Nat+ Organs in Hcv Negative Recipients with On-site Specialty Pharmacy Services: A Win for the Patient, the Transplant Center, and Society? [abstract]. Am J Transplant. 2021; 21 (suppl 3). https://atcmeetingabstracts.com/abstract/the-use-of-hcv-nat-organs-in-hcv-negative-recipients-with-on-site-specialty-pharmacy-services-a-win-for-the-patient-the-transplant-center-and-society/. Accessed February 25, 2026.« Back to 2021 American Transplant Congress