The Trajectory of Donor-Derived Cell-Free DNA (dd-cfDNA) Complements the Baseline Measurements in Heart Transplant Surveillance for Rejection
1Inova Heart and Vascular Institute, Falls Church, VA, 2University of Washington Heart Institute, Seattle, WA, 3Pennsylvania State University, Hershey, PA, 4University of Nebraska Medical Center, Omaha, NE, 5CareDx, Brisbane, CA, 6Stanford University, Stanford, CA, 7University of Southern California, Los Angeles, CA
Meeting: 2022 American Transplant Congress
Abstract number: 235
Keywords: Heart transplant patients, Monitoring, Multicenter studies, Rejection
Topic: Clinical Science » Heart » 63 - Heart and VADs: All Topics
Session Information
Session Name: Heart and VADs: All Topics II
Session Type: Rapid Fire Oral Abstract
Date: Monday, June 6, 2022
Session Time: 3:30pm-5:00pm
 Presentation Time: 3:40pm-3:50pm
Presentation Time: 3:40pm-3:50pm
Location: Hynes Room 210
*Purpose: Changes in dd-cfDNA levels over time may identify patients with evolving alloimmune injury, providing complementary information to the donor fraction. We explored the dd-cfDNA trajectories preceding biopsy-proven rejection events among single-organ heart transplant recipients enrolled in the Surveillance HeartCare® Outcomes Registry (SHORE) and undergoing longitudinal surveillance with dd-cfDNA.
*Methods: In the primary analysis, we identified patients with first-time rejection events and at least 3 preceding dd-cfDNA results, including at least one within 30 days of the index biopsy; we then selected the lowest of two earlier measurements to define the patient-specific baseline. Changes between the baseline and index dd-cfDNA result were characterized.
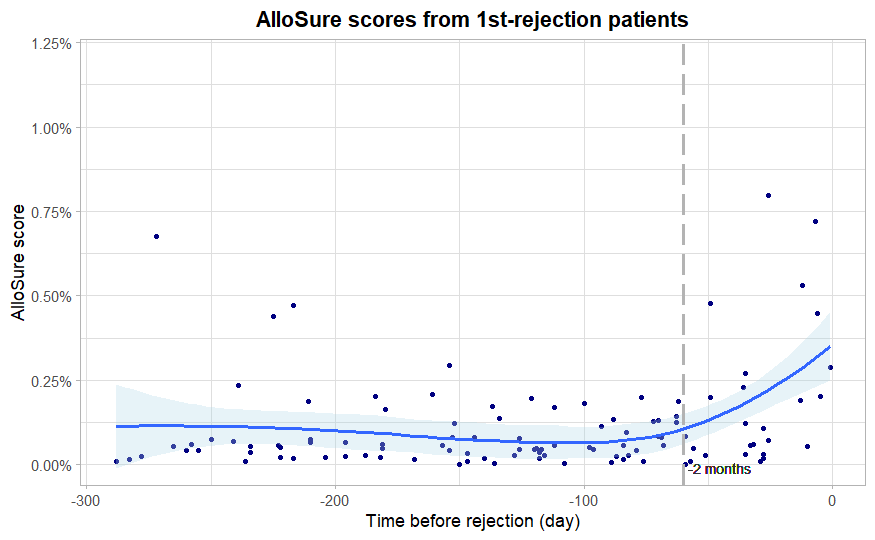
*Results: A total of 25 patients with biopsy-proven rejection met criteria for the initial analysis; among these, median baseline dd-cfDNA was 0.024% (IQR:-0.011% – 0.051%) and median dd-cfDNA at the time of rejection was 0.19% (IQR: 0.054%- 0.590%), representing a 7-fold (IQR 1.5-28.9) increase over a median of 57 (IQR: 42 – 69) days. Further analysis of the longitudinal AlloSure results in these patients demonstrated the dd-cfDNA began to rise about 2 months prior to the rejection episode [Figure 1].
*Conclusions: Longitudinal surveillance with dd-cfDNA allows the integration of changes over time to the individual measurements, allowing earlier identification of evolving allograft injury. Significant changes from an established baseline and between sequential values as much as 2 months prior to biopsy-proven rejection highlight how longitudinal surveillance may improve diagnostic performance.
To cite this abstract in AMA style:
Shah P, Cheng R, Eisen H, Lowes BD, Jenkins LLourenco, Fu Y, Qu K, Teuteberg J, DePasquale E. The Trajectory of Donor-Derived Cell-Free DNA (dd-cfDNA) Complements the Baseline Measurements in Heart Transplant Surveillance for Rejection [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/the-trajectory-of-donor-derived-cell-free-dna-dd-cfdna-complements-the-baseline-measurements-in-heart-transplant-surveillance-for-rejection/. Accessed February 20, 2026.« Back to 2022 American Transplant Congress