The SHELTER Trial of Transplanting Donor Hepatitis C Virus RNA+ Lungs Into Uninfected Recipients
1University of Pennsylvania, Philadelphia, PA, 2University of Miami, Miami, FL
Meeting: 2022 American Transplant Congress
Abstract number: 47
Keywords: Hepatitis C, Lung transplantation, Organ Selection/Allocation
Topic: Clinical Science » Lung » 64 - Lung: All Topics
Session Information
Session Name: Infectious Considerations for Lung Transplantation
Session Type: Rapid Fire Oral Abstract
Date: Sunday, June 5, 2022
Session Time: 3:30pm-5:00pm
 Presentation Time: 4:30pm-4:40pm
Presentation Time: 4:30pm-4:40pm
Location: Hynes Room 210
*Purpose: The advent of direct acting antiviral therapy has motivated centers to transplant lungs from donors with hepatitis C virus infection (HCV-RNA+) into uninfected recipients. Limited data about this practice have been published. SHELTER is a trial of orthotopic lung transplantation using HCV-infected organs for HCV-negative candidates, followed by antiviral treatment (sponsor: Merck; NCT03724149).
*Methods: Single arm trial of 10 lung transplants at a single center. Inclusion criteria include: 40 – 67 years old, waitlisted for lung-only transplant. Exclusions: liver disease; Fibroscan suggesting ≥F2 fibrosis.
*Results: Between 12/2018 and 11/2020, 22 patients consented to screening, among whom 18 were eligible and opted-in for donor HCV-RNA+ lung offers. After waiting a median of 37 days (IQR 6, 373) from UNOS opt-in for HCV-NAT+ donor offers, 10 patients received double lung transplants. Among recipients, the median age was 57 years (IQR 44, 67); 5 (50%) were female and 10 (100%) White race, and 7 (70%) had chronic obstructive pulmonary disease. Median lung allocation score at transplant 34.3 (32.7, 86.9). Median donor age 38 years (IQR 31, 36).
Five had primary graft dysfunction grade 3 or above on day 2 or 3 post-transplant. None received ECMO.
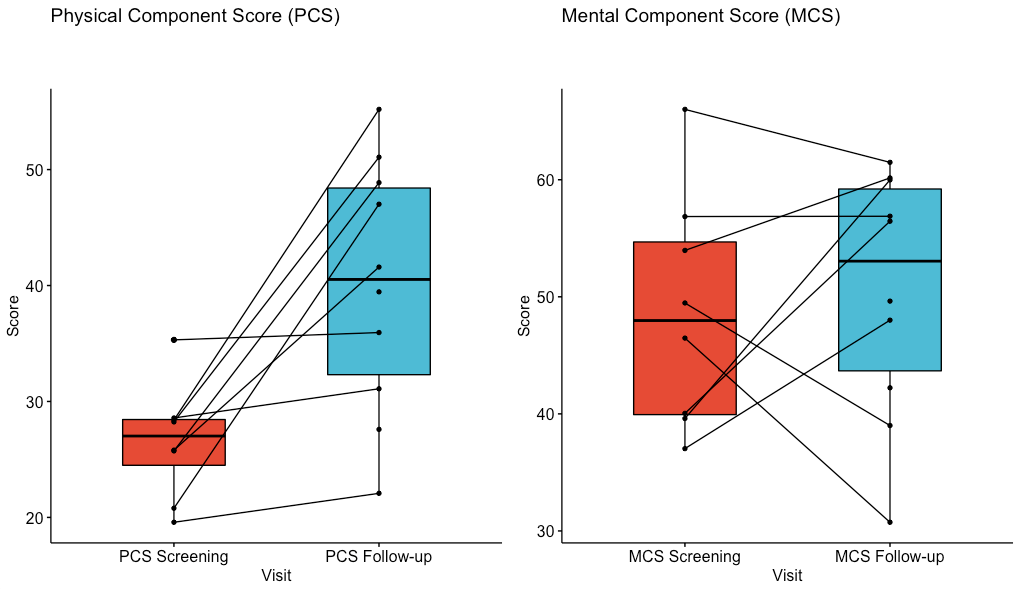
Nine patients received elbasvir and grazoprevir, while 1 received sofosbuvir/velpatasvir (for HCV genotype 3). All 10 patients were cured of HCV, defined as sustained virologic response 12 weeks (SVR-12) after end of therapy. All 10 survived to one year (vs. 86% 1-year survival overall at the center during this period). Serious adverse events were not considered related to HCV or treatment. These serious events included 1) one patient with kidney injury requiring months of dialysis; 2) one patient had grade 3 acute cellular rejection which was responsive to treatment. 3) two patients had donor specific antibodies, class II, without antibody mediated rejection. Figure 1 shows improvement in physical and mental health quality of life scores from pre-transplant to 12 months, assessed with the validated RAND-36 instrument.
*Conclusions: HCV-negative candidates who underwent lung transplant with HCV-RNA+ donor organs responded well to antiviral therapy and had acceptable 1-year outcomes. The SHELTER trial also revealed new and reassuring data about quality of life after transplant using HCV-RNA+ lungs.
To cite this abstract in AMA style:
Potluri V, Reese P, Prenner S, Mentch H, Goldberg D, Claridge T, Smith J, Gentile C, Deerlin VVan, Reddy R, Fallah T, Bermudez C, Crespo M, Diamond J. The SHELTER Trial of Transplanting Donor Hepatitis C Virus RNA+ Lungs Into Uninfected Recipients [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/the-shelter-trial-of-transplanting-donor-hepatitis-c-virus-rna-lungs-into-uninfected-recipients/. Accessed February 24, 2026.« Back to 2022 American Transplant Congress