The Safety of Surveillance and Indication Biopsies in Kidney Transplantation: A Series of 2000 Biopsies
1Internal Medicine/Nephrology, Univ of Michigan Medical Ctr, Ann Arbor, MI, 2Internal Medicine/Nephrology, Univ of California Davis, Sacremento, CA, 3Internal Medicine/Nephrology, Kansas University Medical Center, Kansas City, KS
Meeting: 2019 American Transplant Congress
Abstract number: 39
Keywords: Biopsy, Kidney transplantation, Safety, Screening
Session Information
Session Name: Concurrent Session: Kidney Technical
Session Type: Concurrent Session
Date: Sunday, June 2, 2019
Session Time: 2:30pm-4:00pm
 Presentation Time: 3:42pm-3:54pm
Presentation Time: 3:42pm-3:54pm
Location: Room 304
*Purpose: Kidney biopsy (KB) remains the most important diagnostic tool in kidney transplantation. Despite its value, surveillance KB are not routinely done at majority of transplant centers due to concern for KB complications. We evaluated the overall safety of all KBs performed at a large Midwestern transplant center.
*Methods: We collected data on all surveillance (performed at 3, 6 and 12 months post-transplant) and indication biopsies from 3/31/15 and 10/30/18. Anticoagulation or anti-platelet therapy was held >5 days prior to biopsy when feasible. Blood pressures (BP) needed to be <150/90 mmHg to proceed with biopsy. Patients had hemoglobin (Hb) measured immediately prior to the procedure and 3 hours post-procedure. All patients were observed for 4 hours post-procedure and reassessed for change in Hb, clear urine, absence of pain and acceptable BP prior to discharge home. All biopsies were performed using real time ultrasound localization, 18-gauge Monopty biopsy needles and performed in one of two nephrology-based procedure units.
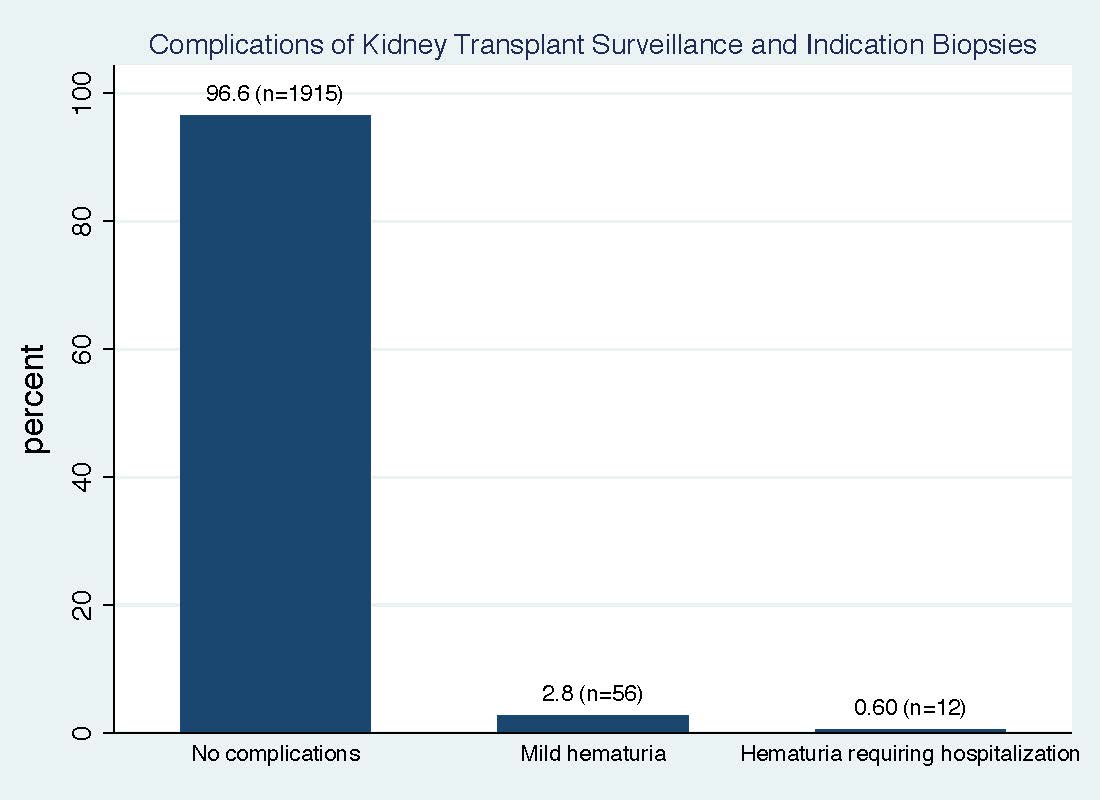
*Results: Among 2018 patients presenting for KB, 1.73% (n=35) were aborted due to presence of fluid, bowel, blood vessels, elevated INR or hydronephrosis). A total of 1983 patients had a KB attempted. The mean pre-procedure Hb was 12.3 +/- 1.8 range (6.8 to 19.1) and mean post Hb was 11.8 +/- 1.8 (6.6 to 18.3). The mean reduction in Hb was 0.6 +/- 0.6 (range -2.8 to 3.1). A total of 68 patients (3.3 %) had gross hematuria with n=12 (0.6%) needing hospitalization for observation of hematuria resolution (figure 1). Four patients needed 3-way bladder catheter irrigation, were admitted for hematuria resolution and one required urology for evacuation of bladder clots. No patients needed blood transfusion. There were no deaths. There was no difference in any complications by history of anticoagulation or anti-platelet use before biopsy.
*Conclusions: KB remains the standard of care diagnostic tool in kidney transplantation. KB can be performed safely, and consideration should be given to expanding the frequency of surveillance biopsies.
To cite this abstract in AMA style:
Naik AS, Huang Y, Cibrik DM, Shaban E, McGuire M, Boyer C, Norman SP. The Safety of Surveillance and Indication Biopsies in Kidney Transplantation: A Series of 2000 Biopsies [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/the-safety-of-surveillance-and-indication-biopsies-in-kidney-transplantation-a-series-of-2000-biopsies/. Accessed February 19, 2026.« Back to 2019 American Transplant Congress