The Risk of Systematic Error in Liver Transplant Opioid Research
J. N. Fleming, N. A. Pilch, S. Ball, P. K. Baliga, D. DuBay, D. J. Taber
Medical University of South Carolina, Charleston, SC
Meeting: 2019 American Transplant Congress
Abstract number: 180
Keywords: Liver transplantation, Screening
Session Information
Session Name: Concurrent Session: Psychosocial and Treatment Adherence
Session Type: Concurrent Session
Date: Sunday, June 2, 2019
Session Time: 4:30pm-6:00pm
 Presentation Time: 4:30pm-4:42pm
Presentation Time: 4:30pm-4:42pm
Location: Room 208
*Purpose: Opioid use before and after liver transplant is strongly associated with morbidity and mortality; yet data assessing opioid utilization has centered on sources with known inaccuracies. The purpose of this study was to evaluate the accuracy of various opioid use data sources compared to state-required opioid prescription data (PDMP, gold-standard), and to determine the impact that the source has on measured outcomes.
*Methods: This was a retrospective, single-center cohort study of opioid use in liver transplant recipients between 2010-6 assessing associated readmission outcomes. Opioid prescription data was obtained via medication reconciliation, a national pharmaceutical claims database, and the state-mandated PDMP.
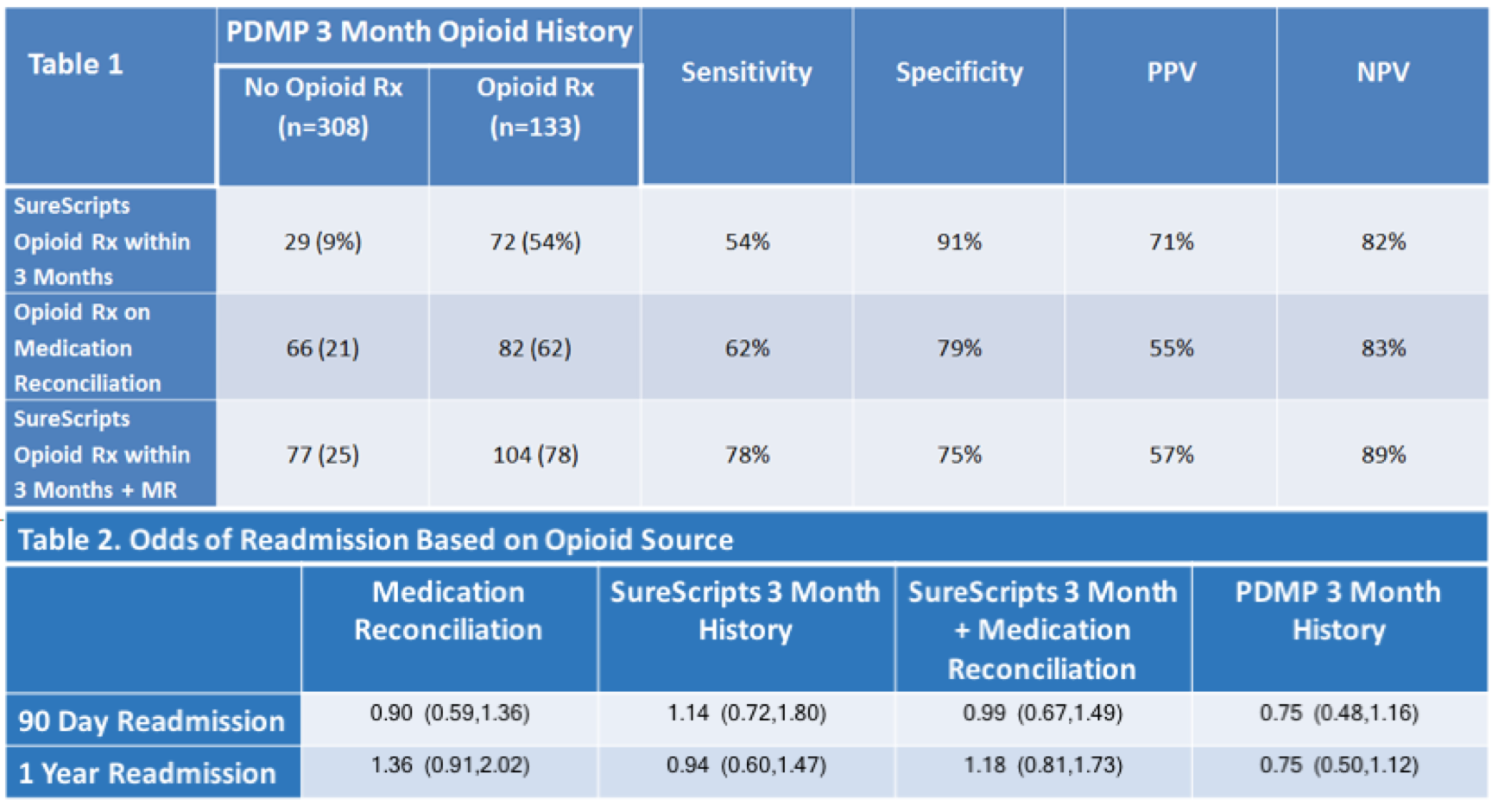
*Results: Of 441 liver transplants within the study timeframe, 133 (30%) had PDMP-reported opioid prescriptions filled in the 3 months prior to transplant. Other opioid sources or combinations of opioid sources were able to accurately identify patients that had used opioids 54 to 78% of the time, yet also inaccurately identified 9 to 25% of patients (who did not fill a prescription for opioids during the time period) as opioid users (Table 1). The associations between pre-txp opioid use and readmission rates varied significantly based on source of information used to assess opioid use (Table 2).
*Conclusions: In order to most optimally mitigate the opioid epidemic, researchers must be able to use the most accurate opioid source data to measure utilization and determine risk factors for COU. It is imperative for researchers to have the ability to match state PDMP data with patient-level characteristics to identify at risk patients within this population. We strongly encourage healthcare workers and researchers to work with their state-level politicians to make this possible.
To cite this abstract in AMA style:
Fleming JN, Pilch NA, Ball S, Baliga PK, DuBay D, Taber DJ. The Risk of Systematic Error in Liver Transplant Opioid Research [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/the-risk-of-systematic-error-in-liver-transplant-opioid-research/. Accessed February 22, 2026.« Back to 2019 American Transplant Congress