The Net State of Immunosuppression in Septuagenarian Liver Transplant Recipients: Balancing Rejection and Infection Risks
1Pharmacy, University of Maryland Medical Center, Baltimore
2University of Maryland School of Medicine, Baltimore.
Meeting: 2018 American Transplant Congress
Abstract number: C212
Keywords: Elderly patients, Immunosuppression, Infection, Liver transplantation
Session Information
Session Name: Poster Session C: Liver: Immunosuppression and Rejection
Session Type: Poster Session
Date: Monday, June 4, 2018
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall 4EF
Purpose: Careful patient selection and perioperative management have allowed for successful liver transplantation in elderly patients. Elderly patients may be more susceptible to the infectious complications of immunosuppression; however, there is a paucity of data describing rejection and infection rates in this population. The purpose of this study is to describe the rejection and infection rates in a septuagenarian liver transplant population in the Share 35 allocation era.
Methods: This was a single-center, retrospective chart review of all liver transplants performed in recipients aged 70 years or greater between 06/2013 and 02/2017. Immunosuppression regimens, biopsy information, and positive culture data within the first 6 months of transplant were collected.
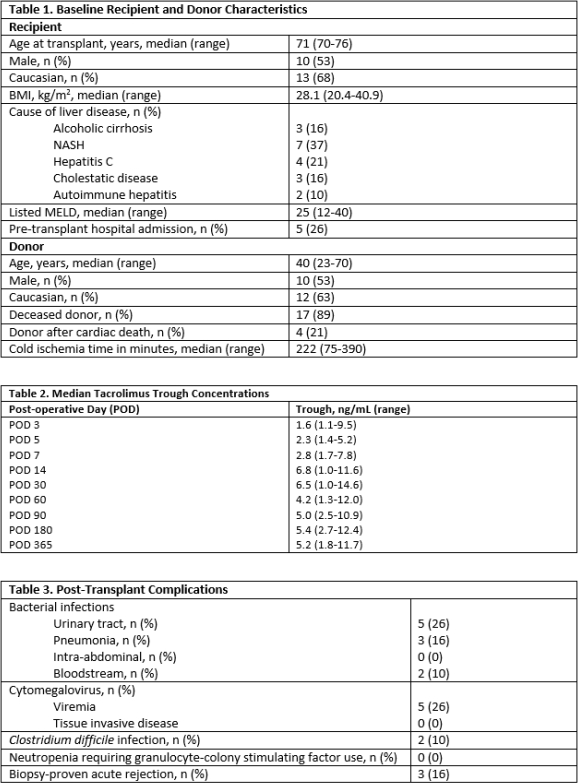
Results: A total of 19 patients were identified, with a median duration of follow up of 336 days. Baseline recipient and donor characteristics are listed in Table 1. The majority of patients (74%) received induction with steroids alone, while 5 patients additionally received basiliximab. Triple maintenance immunosuppression with tacrolimus dosed to target troughs of 4-6 ng/mL, mycophenolate 1000 mg/day, and a 3-week steroid taper was initially employed in all patients, with tacrolimus starting at a median time of post-operative day (POD) 5. Median tacrolimus troughs are listed in Table 2. Post-transplant complications are outlined in Table 3. Rejection occurred early post-transplant in 3 patients at a median time of POD18 (range 11-62). The maximum Banff score was 5 and all rejection episodes were treated with intravenous steroids. Median time to first infection was 35 days post-transplant (range 6-131). There were 4 patient deaths within the first year of transplant, 2 of which were secondary to sepsis.
Conclusions: In carefully selected patients, liver transplantation after the age of 70 is a viable option in the Share 35 era. The natural immunosenescence occurring with advanced age should allow for reduced immunosuppression to minimize infectious complications in this population.
CITATION INFORMATION: Casale J., Clark J., Ravichandran B., LaMattina J. The Net State of Immunosuppression in Septuagenarian Liver Transplant Recipients: Balancing Rejection and Infection Risks Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Casale J, Clark J, Ravichandran B, LaMattina J. The Net State of Immunosuppression in Septuagenarian Liver Transplant Recipients: Balancing Rejection and Infection Risks [abstract]. https://atcmeetingabstracts.com/abstract/the-net-state-of-immunosuppression-in-septuagenarian-liver-transplant-recipients-balancing-rejection-and-infection-risks/. Accessed February 19, 2026.« Back to 2018 American Transplant Congress