The Impact of the Influenza Vaccine on Pediatric Kidney Transplant Outcomes
1Department of Pediatrics, University of Minnesota, Minneapolis, MN
2Fairview Health Services, Minneapolis, MN
3Department of Surgery, University of Minnesota, Minneapolis, MN.
Meeting: 2018 American Transplant Congress
Abstract number: B213
Keywords: Infection, Kidney transplantation, Pediatric, Vaccination
Session Information
Session Name: Poster Session B: Kidney: Pediatrics
Session Type: Poster Session
Date: Sunday, June 3, 2018
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall 4EF
The influenza vaccine has been associated with de novo DSA and rejection in adult kidney recipients. This risk has not been adequately assessed in pediatric kidney recipients. In a single-center retrospective analysis of pediatric kidney recipients, we assessed graft and patient outcomes stratified by whether the patients received the influenza vaccine. From January 1, 2006 to December 31, 2015, 125 of 187 patients received the influenza vaccine within one year post-transplant. There were no significant differences in baseline characteristics between groups (values presented as N (%), Mean +/- SD or Median (IQR)).
| Vaccinated n=125 | Not vaccinated n=62 | P value | |
| Female | 48 [38%] | 30 [48%] | 0.25 |
| Recipient age | 9.5 ± 0.6 | 10.8 ± 5.3 | 0.14 |
| Donor age | 31.9 ± 11.0 | 33.2 ± 10.7 | 0.44 |
| Pre-transplant peak PRA I | 3.00 [0.00; 31.0] | 2.00 [0.00; 16.8] | 0.08 |
| Pre-transplant peak PRA II | 2.00 [0.00; 34.0] | 0.00 [0.00; 6.00] | 0.32 |
Kaplan-Meier curves and log-rank tests were used to compare mortality, graft failure and rejection. Due to the timing of the vaccine, baseline was defined as one year post-transplant; those with events within one year of transplant had an event time of zero. The vaccinated group had a significantly decreased risk of mortality (p=0.048) after one year post-transplant. 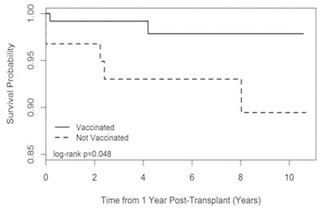 There was no difference in death-censored graft survival (p=0.253), graft survival (p=0.098), or rejection (p=0.195). Eight patients developed de novo DSA within 3 months post-vaccination; of these, 4 had persistent DSA for over 2 months. Of these 8 patients, 3 developed biopsy-proven rejection; 2 had persistent DSA, and 1 had DSA that resolved prior to rejection. In our cohort of pediatric kidney recipients, the influenza vaccine was safe; larger prospective studies are required to confirm that the vaccine is not associated with increased de novo DSA and rejection risk in pediatric kidney recipients.
There was no difference in death-censored graft survival (p=0.253), graft survival (p=0.098), or rejection (p=0.195). Eight patients developed de novo DSA within 3 months post-vaccination; of these, 4 had persistent DSA for over 2 months. Of these 8 patients, 3 developed biopsy-proven rejection; 2 had persistent DSA, and 1 had DSA that resolved prior to rejection. In our cohort of pediatric kidney recipients, the influenza vaccine was safe; larger prospective studies are required to confirm that the vaccine is not associated with increased de novo DSA and rejection risk in pediatric kidney recipients.
CITATION INFORMATION: Camerino M., Jackson S., Chinnakotla S., Verghese P. The Impact of the Influenza Vaccine on Pediatric Kidney Transplant Outcomes Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Camerino M, Jackson S, Chinnakotla S, Verghese P. The Impact of the Influenza Vaccine on Pediatric Kidney Transplant Outcomes [abstract]. https://atcmeetingabstracts.com/abstract/the-impact-of-the-influenza-vaccine-on-pediatric-kidney-transplant-outcomes/. Accessed March 9, 2026.« Back to 2018 American Transplant Congress
