The Impact of Tacrolimus IPV and TTR on the Development of De Novo Donor-specific Antibodies in Kidney Transplant Recipients
1Pharmacy, Beth Israel Deaconess Medical Center, Boston, MA, 2Nephrology, Beth Israel Deaconess Medical Center, Boston, MA, 3Pathology, Beth Israel Deaconess Medical Center, Boston, MA
Meeting: 2021 American Transplant Congress
Abstract number: 822
Keywords: FK506, HLA antibodies, Kidney transplantation, Rejection
Topic: Clinical Science » Pharmacy » Non-Organ Specific: Pharmacogenomics / Pharmacokinetics
Session Information
Session Name: Non-Organ Specific: Pharmacogenomics / Pharmacokinetics
Session Type: Poster Abstract
Session Date & Time: None. Available on demand.
Location: Virtual
*Purpose: High intrapatient variability (IPV) and low time in therapeutic range (TTR) of tacrolimus (TAC) may increase the risk of developing de novo donor-specific antibodies (dnDSA) among transplant recipients. The purpose of the study was to assess: (1) mean IPV and TTR between patients who developed dnDSA versus patients who did not (control), 2) rates of biopsy proven rejection, and 3) death-censored graft loss.
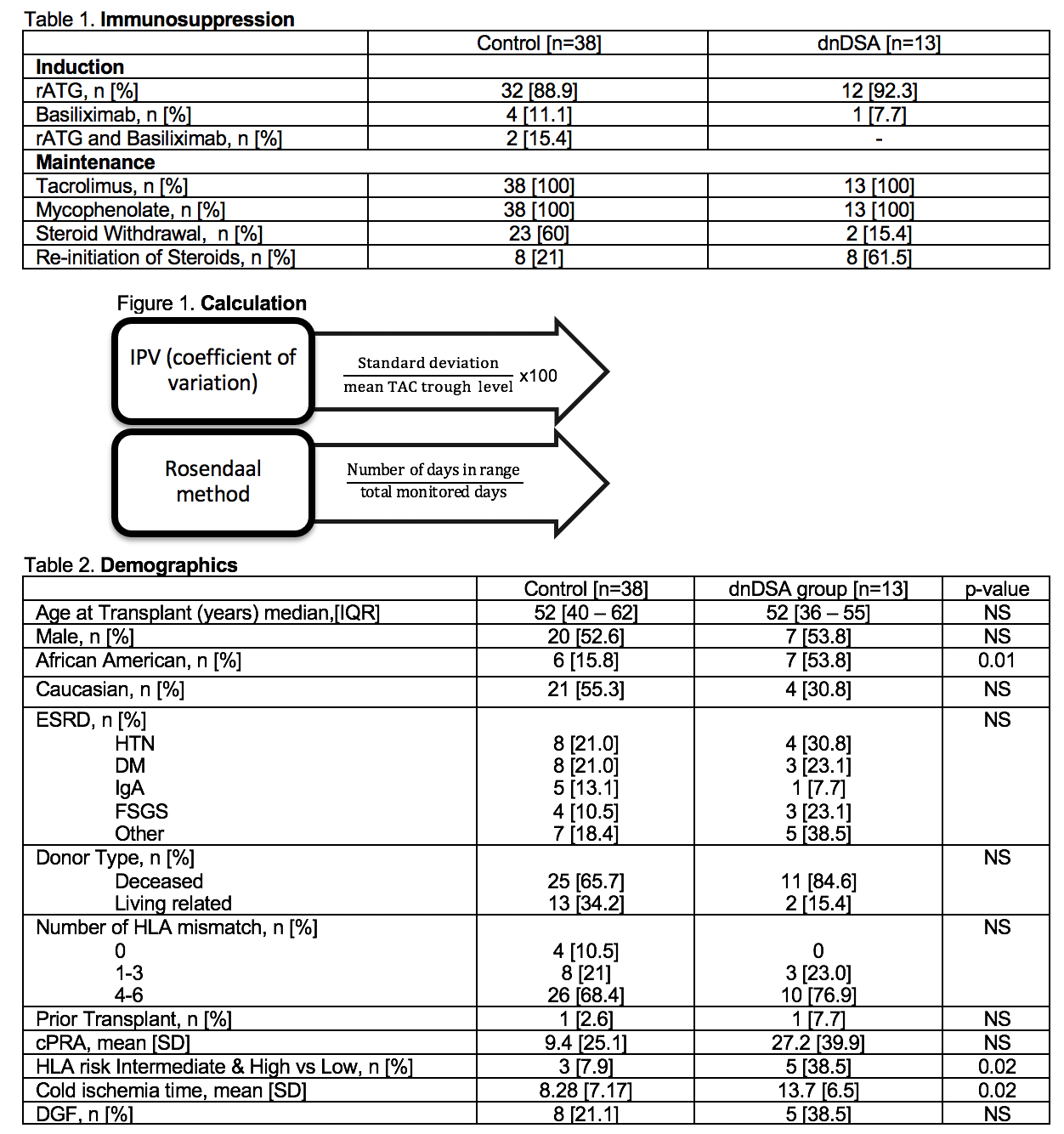
*Methods: This is a retrospective, single center chart review of 51 adults who underwent renal transplantation between January 2014-December 2018. All patients had 2 years of post-transplant follow-up data available. Induction and maintenance immunosuppression is described in Table 1. Patients were excluded if they failed to undergo DSA screening post-transplant or received a multi-organ transplant. Methods for calculations of IPV and TTR are summarized in Figure 1.
*Results: Patients who did not develop dnDSA “Control” (n=38) and those who developed dnDSA (n=13) were compared. Baseline characteristics are described in Table 2. Based on institutional protocol, the majority of patients received rATG and approximately half underwent steroid withdrawal. The mean IPVs and TTRs were not statistically significant when comparing the 2 groups, but there was a trend towards significance for TTRs at 12 months (55.6±19.6 vs 45±21.7, p=0.08) (Table 3 and 4). Rates of biopsy proven rejection were significantly higher in the dnDSA group (38.5% vs 5.2%, p=0.008). 1 patient in dnDSA group experienced death-censored graft loss due to antibody mediated rejection. When evaluating IPV and TTR of patients who developed dnDSA and rejection, the results were concurrent with current literature (Table 5).
*Conclusions: Mean IPVs between control and dnDSA groups were not significantly different at all time points. Although the differences in TTR did not reach statistical significance, patients in the dnDSA group had a trend towards lower TTR. This suggests that TTR may have more impact than IPV in predicting dnDSA development following renal transplantation.
To cite this abstract in AMA style:
Xu D, Richards K, Zheng K, Patel H, Cardarelli F, Pena JA, Aala A. The Impact of Tacrolimus IPV and TTR on the Development of De Novo Donor-specific Antibodies in Kidney Transplant Recipients [abstract]. Am J Transplant. 2021; 21 (suppl 3). https://atcmeetingabstracts.com/abstract/the-impact-of-tacrolimus-ipv-and-ttr-on-the-development-of-de-novo-donor-specific-antibodies-in-kidney-transplant-recipients/. Accessed March 6, 2026.« Back to 2021 American Transplant Congress