The Banff Classification of Antibody-Mediated Rejection in Kidney Allografts: Comparison of Predictive Performance for Allograft Failure in Previous and Current Editions
1Microbiology, Immunology and Transplantation, KU Leuven, Leuven, Belgium, 2Histocompatibility and Immunogenetics Laboratory, Red Cross-Flanders, Mechelen, Belgium, 3Imaging and Pathology, KU Leuven, Leuven, Belgium
Meeting: 2020 American Transplant Congress
Abstract number: 22
Keywords: Antibodies, Kidney transplantation, Prognosis, Rejection
Session Information
Session Name: Kidney Acute Antibody Mediated Rejection
Session Type: Oral Abstract Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:45pm
 Presentation Time: 3:51pm-4:03pm
Presentation Time: 3:51pm-4:03pm
Location: Virtual
*Purpose: Since its introduction in 2001, the Banff classification for antibody-mediated rejection (ABMR) has undergone significant changes, mainly by inclusion of C4d-negative ABMR in 2013 and removal of suspicious ABMR (sABMR) with the use of C4d as surrogate for DSA in 2017. It is unclear to what extent these changes have altered the prognostic value of the Banff classification.
*Methods: In a single-center cohort study of 1000 single kidney transplantations between 2004 and 2013, all 3470 protocol and 847 indication biopsies were classified according to the 2001, 2013 and 2017 Banff classification. Survival analysis was based on the most severe rejection category occurring within the first year after transplantation.
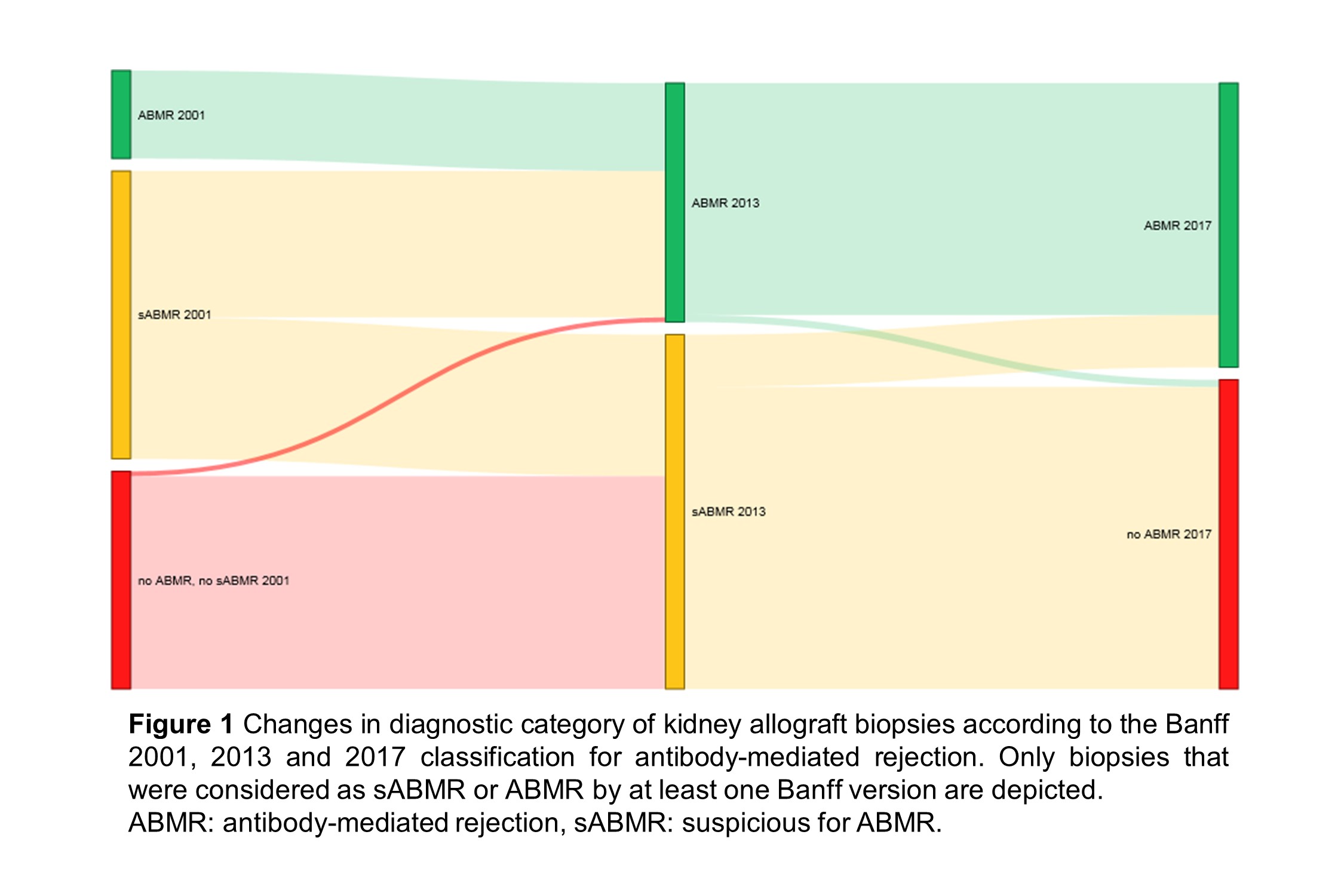
*Results: Of the 4317 biopsies included, 499 were defined as ABMR or sABMR by at least one Banff edition. Comparing the 2001 and 2013 classifications, sABMR and ABMR diagnoses increased from 242 to 298 biopsies, and from 74 to 201 biopsies, respectively (Figure 1). The hazard ratio for allograft failure (95% CI) after ABMR within the first year increased from 3.79 (1.96-7.33) to 4.25 (2.60-6.96). In the Banff 2017 update, removal of the suspicious category caused reclassification of 254/298 sABMR biopsies to No ABMR, and 44/298 to ABMR. However, overall HR after ABMR decreased to 3.47 (2.23-5.41), since reclassified sABMR cases associated with better survival than biopsies considered as ABMR in previous Banff editions. Conversely, sABMR reclassified to No ABMR conferred worse outcome than biopsies previously considered as absence of ABMR.
*Conclusions: Inclusion of C4d-negative ABMR in the 2013 Banff revision importantly increased the numbers of cases with ABMR and augmented the predictive performance for allograft outcome. In contrast, removal of the suspicious category and introduction of DSA-negative ABMR in 2017 decreased the prognostic value of the Banff classification. These data demonstrate the clinical relevance of an intermediate ABMR category, of which the reintroduction should be considered in future updates.
To cite this abstract in AMA style:
Callemeyn J, Ameye H, Coemans M, Senev A, Lerut E, Sprangers B, Kuypers D, Emonds M, Naesens M. The Banff Classification of Antibody-Mediated Rejection in Kidney Allografts: Comparison of Predictive Performance for Allograft Failure in Previous and Current Editions [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/the-banff-classification-of-antibody-mediated-rejection-in-kidney-allografts-comparison-of-predictive-performance-for-allograft-failure-in-previous-and-current-editions/. Accessed March 1, 2026.« Back to 2020 American Transplant Congress