Tacrolimus Time in Therapeutic Range Predicts Adverse Clinical Outcomes in the First Year of Kidney Transplant.
University of Colorado, Aurora, CO
Meeting: 2017 American Transplant Congress
Abstract number: 74
Keywords: HLA antibodies, Immunosuppression, Kidney transplantation, Rejection
Session Information
Session Name: Concurrent Session: Novel Immunosuppression Regimens - Tacrolimus Combinations
Session Type: Concurrent Session
Date: Sunday, April 30, 2017
Session Time: 2:30pm-4:00pm
 Presentation Time: 3:42pm-3:54pm
Presentation Time: 3:42pm-3:54pm
Location: E450b
Tacrolimus (TAC) has a narrow therapeutic range, with its upper bounds limited by serious toxicities while TAC minimization strategies have demonstrated immunologic risk. Monitoring the duration of time that a patient is in therapeutic range (TTR) is an effective tool for optimizing the safety and efficacy of drug therapy. The purpose of this study was to apply TTR to TAC therapy to predict increased risk of adverse clinical outcomes.
From 2007 to 2013, kidney transplant recipients who were initiated and maintained on TAC in the first year of transplant were prospectively screened for dnDSA at months 1, 6, 12, and when clinically indicated. TAC troughs after dnDSA or acute rejection were excluded. The Rosendaal method was applied to calculate TAC TTR using a therapeutic range of 5 – 10 ng/ml. TTR <60% or <75% was chosen as high risk based on existing studies applying TTR to other drug therapy. Logistic regression was used to calculate odds ratios and Cox's proportional hazards regression for hazard ratios for time to AR and time to death censored graft loss, which were adjusted for HLA mismatches, age, ethnicity, donor type, gender, induction therapy, and delayed graft function.
There were 560 patients included in the analysis. By 12 months, 163 (29.1%) patients had a TTR <60%, 268 (47.9%) patients with TTR <75%, 132 (23.6%) patients had dnDSA, and there were 42 (7.5%) episodes of acute rejection. There was an increased risk of dnDSA at 12 months for a TTR <60% (OR 1.97, 95% CI 1.26-3.07, p=0.003) and a TTR <75% (OR 1.59, 95% CI 1.04-2.43, p=0.032), acute rejection by 12 months (TTR <60%: HR 3.17, 95% CI 1.70-5.91, p<0.001; TTR <75%: HR 3.19, 95% CI 1.59-6.42, p=0.001), and death-censored graft loss by 5 years (TTR <60%: HR 3.82, 95% CI 1.92-7.60, p<0.001; TTR <75%: HR 2.34 95% CI 1.17-4.69, p=0.016).
A TAC TTR of <60% and <75% was associated with increased risk of dnDSA and acute rejection in the first year of kidney transplant and with graft loss by 5 years. This novel approach to monitoring TAC therapy may be a useful tool to identify patients at high risk for adverse outcomes and warrants further investigation.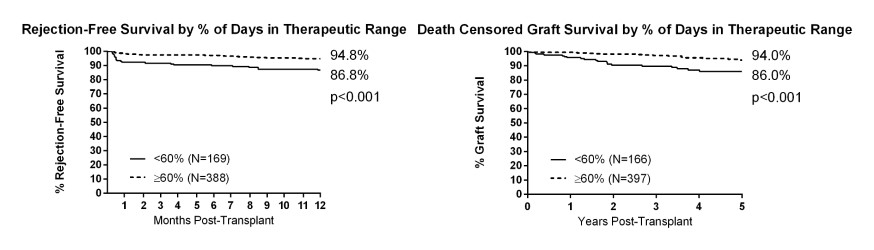
CITATION INFORMATION: Davis S, Gralla J, Klem P, Wiseman A, Cooper J. Tacrolimus Time in Therapeutic Range Predicts Adverse Clinical Outcomes in the First Year of Kidney Transplant. Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Davis S, Gralla J, Klem P, Wiseman A, Cooper J. Tacrolimus Time in Therapeutic Range Predicts Adverse Clinical Outcomes in the First Year of Kidney Transplant. [abstract]. Am J Transplant. 2017; 17 (suppl 3). https://atcmeetingabstracts.com/abstract/tacrolimus-time-in-therapeutic-range-predicts-adverse-clinical-outcomes-in-the-first-year-of-kidney-transplant/. Accessed February 19, 2026.« Back to 2017 American Transplant Congress
