Tacrolimus Intra-Patient Variability Determined from Daily Trough Levels in Adherent Kidney and Liver Transplant Recipients.
A. Leino,1 T. Kaiser,1 Y. Zou,2 E. King,2 A. Vinks,2 W. Jiang,4 U. Christians,3 E. Woodle,1 R. Alloway.1
1University of Cincinnati, Cincinnati
2Cincinnati Children's Hospital, Cincinnati
3University of Colorado, Denver
4Food and Drug Administration, Silver Spring
Meeting: 2017 American Transplant Congress
Abstract number: 92
Keywords: Immunosuppression, Kidney transplantation, Liver transplantation, Pharmacokinetics
Session Information
Session Name: Concurrent Session: Psychosocial and Mediation Adherence Concerns
Session Type: Concurrent Session
Date: Sunday, April 30, 2017
Session Time: 2:30pm-4:00pm
 Presentation Time: 3:06pm-3:18pm
Presentation Time: 3:06pm-3:18pm
Location: E271b
Drugs are classified as highly variable when intra-patient coefficient of variation (CV%) is > 30%. Tacrolimus (TAC) is characterized as highly variable often without supporting evidence. Literature reports variability to be associated with nonadherence and poor outcomes despite potential influencing factors such as TAC formulation. TAC trough variability was reported as 11.3-12.2% and 12.0-12.9% in kidney and liver recipients, respectively in controlled settings. The purpose of this study was to prospectively determine the CV% of daily TAC trough concentrations in adherent patients receiving 3 TAC formulations.
Daily TAC levels were collected using dry blood spot (DBS) technology over 6 weeks (n=42/patient). Patients were randomized to each formulation for 2 1-week periods. No changes in TAC dose or concomitant medications occurred. Adherence was monitored by patient diary, pill counts, and Medication Event Monitoring System. Blood was extracted from protein saver cards for analysis by HPLC/MS. Bland-Altman plots compared DBS and venipuncture troughs weekly. Intra-individual variability was assessed as CV%= (SD/mean TAC concentration after log-transformation) x 100 for the 42 DBS trough levels/patient.
Demographics are in table 1. MEMS based adherence was 99.7%. No differences were observed in Bland-Altman plots by formulation or organ type. The CV% for 3 TAC formulations is presented in table 2. The CV% for each formulation was compared using ratio and 90% confidence interval (table 3).
This is the first report of prospectively collected daily TAC levels to quantitate variability in adherent patients. We conclude: 1) In contrast to most literature, TAC did not demonstrate high intra-patient variability; 2) No significant differences in CV% by formulation or organ type were observed, and 3) The difference in CV% between kidney and liver recipients warrant further study. This establishes baseline variability for future adherence studies.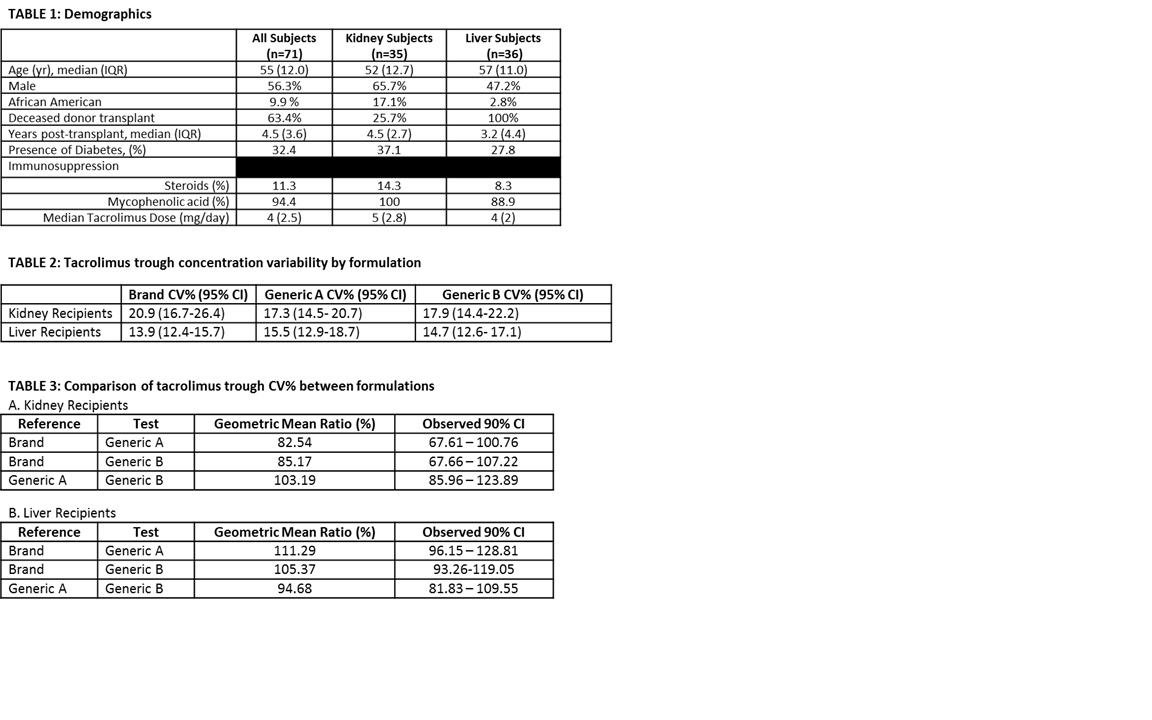
CITATION INFORMATION: Leino A, Kaiser T, Zou Y, King E, Vinks A, Jiang W, Christians U, Woodle E, Alloway R. Tacrolimus Intra-Patient Variability Determined from Daily Trough Levels in Adherent Kidney and Liver Transplant Recipients. Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Leino A, Kaiser T, Zou Y, King E, Vinks A, Jiang W, Christians U, Woodle E, Alloway R. Tacrolimus Intra-Patient Variability Determined from Daily Trough Levels in Adherent Kidney and Liver Transplant Recipients. [abstract]. Am J Transplant. 2017; 17 (suppl 3). https://atcmeetingabstracts.com/abstract/tacrolimus-intra-patient-variability-determined-from-daily-trough-levels-in-adherent-kidney-and-liver-transplant-recipients/. Accessed February 22, 2026.« Back to 2017 American Transplant Congress
