Tabelecleucel (Tab-cel) for Solid Organ (SOT) or Allogeneic Hematopoietic Cell Transplant (HCT) Recipients with Epstein-Barr Virus-Driven Post Transplant Lymphoproliferative Disease (EBV+ PTLD) After Failure Of Rituximab (R) ± Chemotherapy (CT) (ALLELE)
1Boston Children's Hospital/Dana Farber Cancer Institute, Boston, MA, 2University of Miami/Jackson Memorial Hospital, Miami, FL, 3Sorbonne Université, Hôpital de la Pitié-Salpêtrière, Paris, France, 4University of Maryland School of Medicine, Baltimore, MD, 5Atara Biotherapeutics, South San Francisco, CA, 6UPMC Hillman Cancer Center, Pittsburgh, PA, 7Columbia University Medical Center, NY, NY, 8Ingram Cancer Center, Vanderbilt Health, Nashville, TN, 9Loyola University Medical Center, Chicago, IL, 10MD Anderson Cancer Center, Houston, TX
Meeting: 2022 American Transplant Congress
Abstract number: 59
Keywords: Post-transplant lymphoproliferative disorder (PTLD)
Topic: Clinical Science » Infection Disease » 28 - PTLD: All Topics
Session Information
Session Name: PTLD and Malignancies
Session Type: Rapid Fire Oral Abstract
Date: Sunday, June 5, 2022
Session Time: 3:30pm-5:00pm
 Presentation Time: 3:30pm-3:40pm
Presentation Time: 3:30pm-3:40pm
Location: Hynes Room 309
*Purpose: Tab-cel is an investigational, off-the-shelf, allogeneic EBV-specific T-cell immunotherapy for serious EBV-driven diseases, including EBV+ PTLD. Poor overall survival (OS) in patients (pts) with relapsed/refractory EBV+ PTLD reveals an urgent unmet need for effective therapies. We previously reported promising interim data from ALLELE (NCT03394365), a ph 3 multicenter clinical trial of tab-cel after failure of R±CT in pts with EBV+ PTLD following SOT or HCT. Here we highlight overall SOT efficacy and safety; outcomes for SOT1 (failure of R) and SOT2 (failure of R+CT) subgroups will be presented.
*Methods: Outcomes were assessed in pts with EBV+ PTLD following SOT or HCT after failure of R±CT and in the SOT1 (n=11) and SOT2 (n=13) subgroups. Pts received tab-cel at 2×106 cells/kg on days 1, 8 and 15 in a 35d treatment cycle.
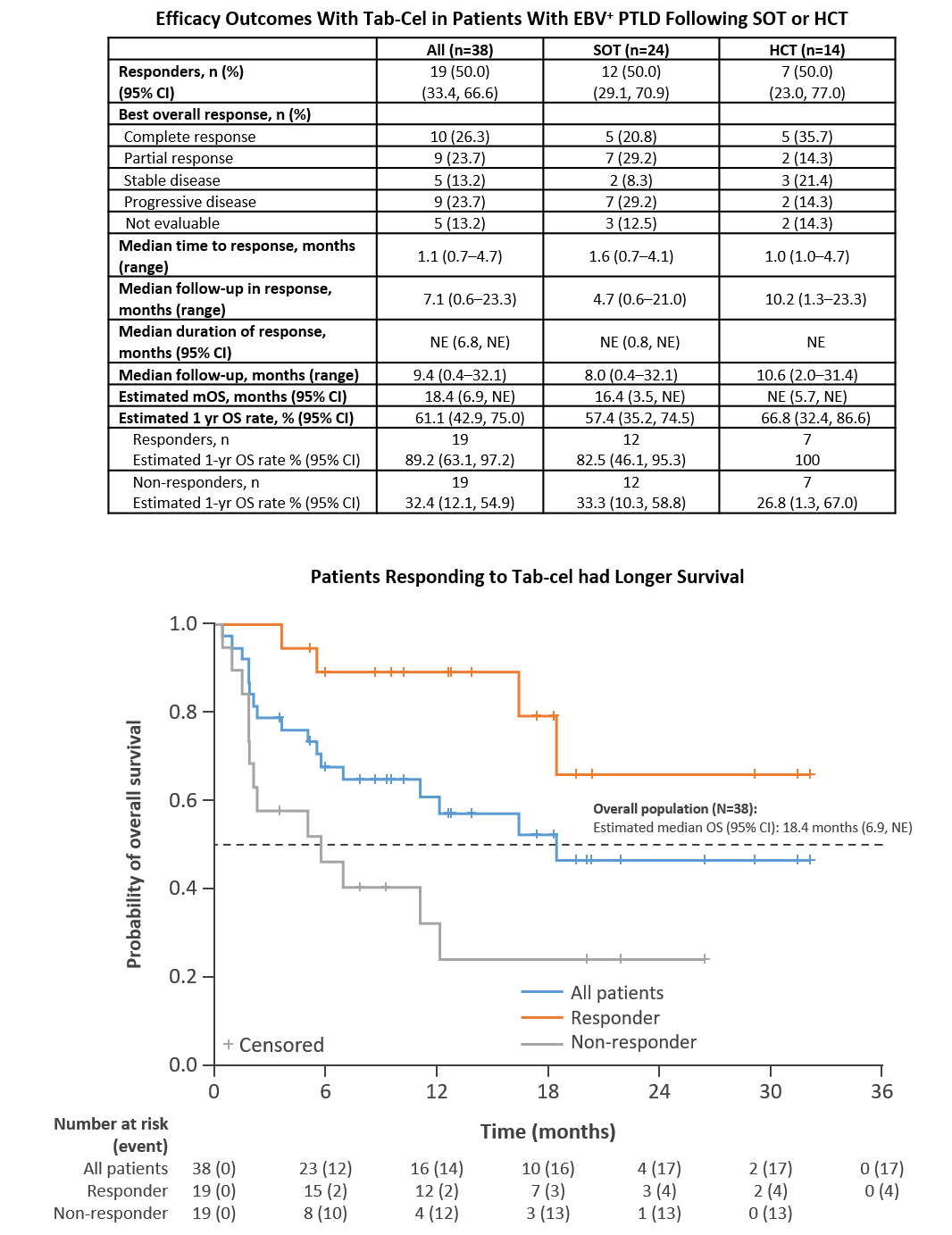
*Results: As of May 2021, 24 SOT and 14 HCT pts were evaluable for assessment and had the opportunity for 6 mo follow-up. Objective response rate (ORR) was 50% (19/38) in all pts and 50% (12/24) in SOT, with best overall response of CR (26.3%) or PR (23.7%) (Table). Overall, median time to response was 1.1 mo; 11/19 responders had a duration of response lasting >6 mo. Estimated median OS was 18.4 mo overall and 16.4 mo for SOT; estimated 1-year survival rates were 61.1% overall and 57.4% for SOT. Responders had a longer survival vs non-responders in overall (Fig) and SOT groups. Further analysis of SOT1 and SOT2 subgroups will be presented. Tab-cel was well-tolerated with no evidence of adverse events attributable to treatment.
*Conclusions: We show clinically meaningful outcomes and promising ORR and OS in a pt population with poor survival and no approved therapies. Tab-cel was well tolerated without evidence of safety concerns typically observed with autologous chimeric antigen receptor cell therapies.
To cite this abstract in AMA style:
Prockop S, Beitinjaneh A, Choquet S, Dahiya S, Dinavahi R, Farah R, Gamelin L, Joshi A, Mehta A, Nayak P, Reshef R, Satyanarayana G, Stiff P, Ye W, Mahadeo KM. Tabelecleucel (Tab-cel) for Solid Organ (SOT) or Allogeneic Hematopoietic Cell Transplant (HCT) Recipients with Epstein-Barr Virus-Driven Post Transplant Lymphoproliferative Disease (EBV+ PTLD) After Failure Of Rituximab (R) ± Chemotherapy (CT) (ALLELE) [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/tabelecleucel-tab-cel-for-solid-organ-sot-or-allogeneic-hematopoietic-cell-transplant-hct-recipients-with-epstein-barr-virus-driven-post-transplant-lymphoproliferative-disease-ebv-ptld-after/. Accessed February 27, 2026.« Back to 2022 American Transplant Congress