T Follicular Regulatory Cell Deficiency Enhances Secondary T Follicular Helper Cell Responses after Transplantation
Emory University, Atlanta, GA
Meeting: 2020 American Transplant Congress
Abstract number: 614
Session Information
Session Name: Lymphocyte Biology: Signaling, Co-Stimulation, Regulation
Session Type: Oral Abstract Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:45pm
 Presentation Time: 4:03pm-4:15pm
Presentation Time: 4:03pm-4:15pm
Location: Virtual
*Purpose: Deleterious alloantibodies result from T follicular helper (Tfh) cell-driven germinal center (GC) reactivity. T follicular regulatory (Tfr) cells are a subset of regulatory T cells that have been identified as important regulators of GC responses and antibody formation. However, whether Tfr cells regulate Tfh cells in vivo is unknown and little is understood of their role in transplantation. Thus given the potentially beneficial impact Tfr cells could have in controlling pathologic donor-specific antibody (DSA) responses, it is of great interest to understand their role in transplantation.
*Methods: To examine the Tfr cell response in transplantation, we first utilized a full MHC mismatch murine skin allograft model to test for and define the kinetics of alloresponsive Tfr cells. We next developed a conditional Tfr knockout (KO, Bcl6fl/flFoxp3Cre) mouse to study the function of Tfr cells in response to full mismatch (BALB/c) and minor antigen (OVA) mismatch skin grafts. The primary and secondary graft-draining lymph node (dLN) Tfh cell, GC and DSA responses in the absence of Tfr cells were evaluated in comparison to wild type littermate (WT, Bcl6fl/flFoxp3wt) controls.
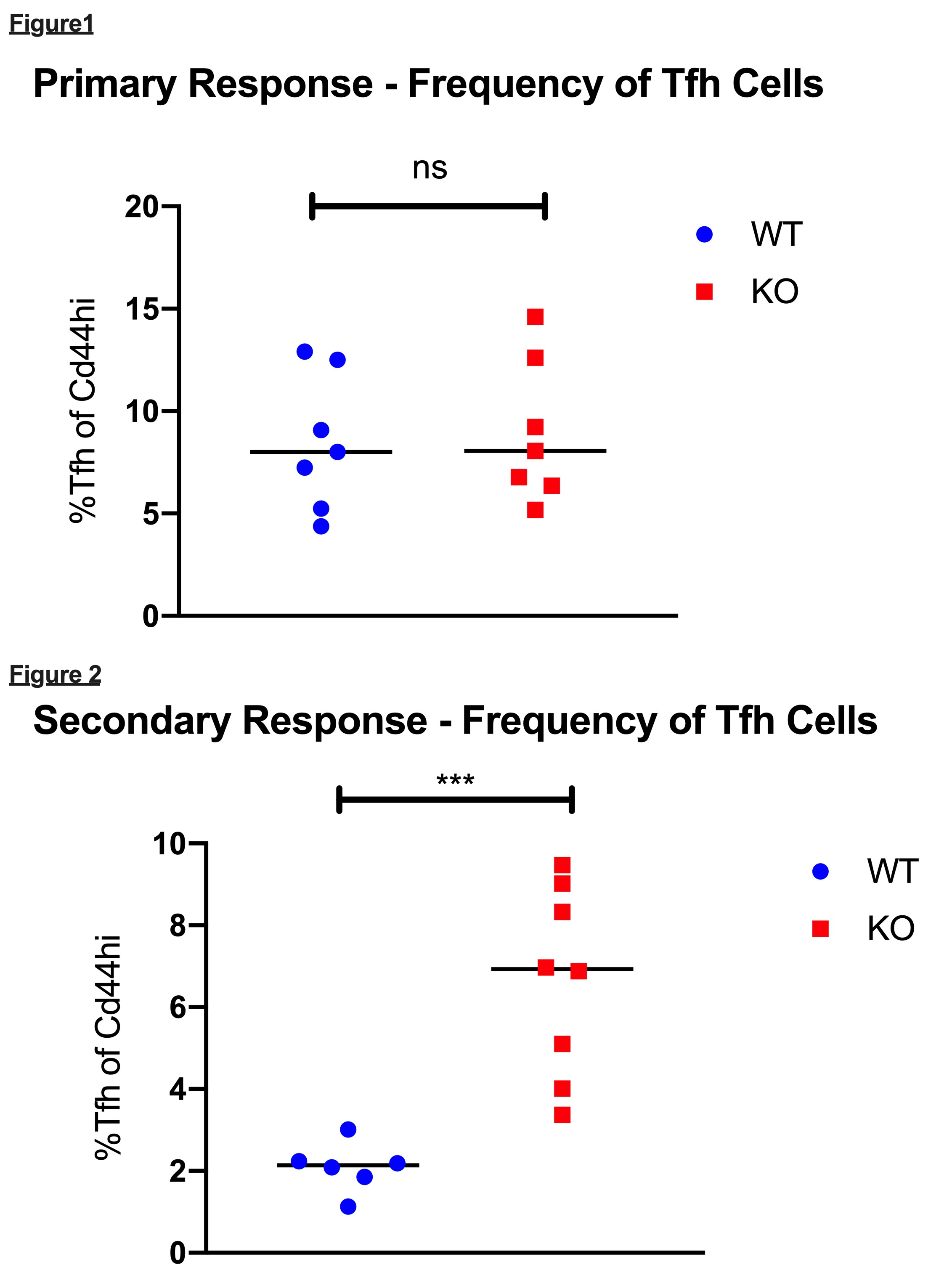
*Results: Tfr cells were detected following transplantation, but the frequency and number of Tfr cells remained stable over time relative to the dynamic expansion and contraction of their Tfh cell counterparts. Tfr KO mice were confirmed by the absence of CD4+CXCR5+PD1hiFoxp3+ T cells in dLNs, spleen and blood. After primary Balb/c and OVA skin grafts, no significant differences were observed in the frequencies of dLN Tfh cell (8.01 vs. 8.05, p=0.805, Figure 1) responses between WT and KO recipient mice. Upon allograft re-challenge with both minor and major mismatch skin transplants, KO mice exhibited a greater frequency of Tfh cells (2.14 vs. 6.93, p=0.001) compared to WT mice in dLNs post-transplant (Figure 2). There were no significant differences in anti-OVA or anti-Balb/c IgG DSA formation between groups during the primary or secondary responses.
*Conclusions: These findings indicate that the absence of Tfr cells does not affect the primary humoral response to an allograft, but rather may impact secondary Tfh cell responses. As such, Tfr cells could potentially be utilized to therapeutically suppress Tfh cell-driven anamnestic humoral alloimmunity.
To cite this abstract in AMA style:
Crichton ES, Zeng S, Badell I. T Follicular Regulatory Cell Deficiency Enhances Secondary T Follicular Helper Cell Responses after Transplantation [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/t-follicular-regulatory-cell-deficiency-enhances-secondary-t-follicular-helper-cell-responses-after-transplantation/. Accessed February 27, 2026.« Back to 2020 American Transplant Congress