Suppressive Effect of De Novo Everolimus on Primary Cytomegalovirus Infection in Kidney Transplant Recipient
1Urology, Sapporo City General Hospital, Sapporo, Japan, 2Kidney Transplant Surgery, Sapporo City General Hospital, Sapporo, Japan
Meeting: 2022 American Transplant Congress
Abstract number: 1373
Keywords: Cytomeglovirus, Immunosuppression
Topic: Clinical Science » Kidney » 37 - Kidney Immunosuppression: Induction Therapy
Session Information
Session Name: Kidney Immunosuppression: Induction Therapy
Session Type: Poster Abstract
Date: Monday, June 6, 2022
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
*Purpose: It has been reported that everolimus (EVR) administration reduces the incidence of cytomegalovirus (CMV) infection, but clinical outcome in CMV-seronegative recipients with EVR-based regimen as immunosuppressive induction therapy is unclear.
*Methods: 41 Patients with CMV-seronegative who underwent living-related kidney transplant (KT) from CMV-seropositive donors were enrolled. Among 41, 25 recipients were treated with EVR in addition to tacrolimus (TAC), mycophenolate mofetil (MMF) and only 3 doses of perioperative methylprednisolone (EVR group). Historical control group was composed of 16 recipients who received TAC/MMF with or without methylprednisolone (Control group). Basiliximab was also used as induction mAb in each group. All CMV-seronegative recipients received valganciclovir(VGCV) for prophylaxis until 4 months post kidney transplantation. The incidence within 1 year and the time of primary CMV infection, the incidence of CMV disease which required ganciclovir(GCV), and the rate of CMV-antibody acquisition were analyzed retrospectively.
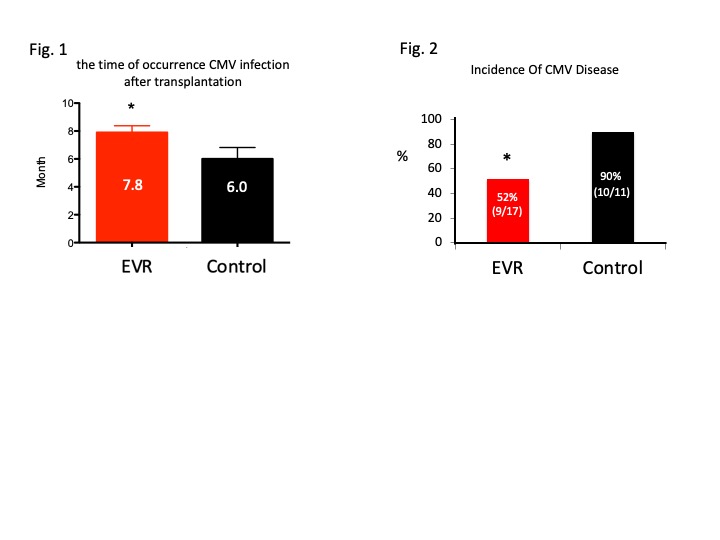
*Results: There were no statistical differences between the two groups in age at transplant, gender, high-risk immunosuppression, incidence of acute rejection within 1 year and prophylactic administration period. The incidence of primary CMV infection within 1 year was 68% for both, while the time of occurrence (months after transplantation) was significantly later in the EVR group (EVR: 7.8 months, Control: 6 months, p=0.04)) (Fig.1). In addition, there were significantly fewer cases of CMV disease requiring GCV with EVR. (EVR: 52%, Control: 90%, p=0.04) (Fig.2). There was also no statisticaldifference in the acquisition rate of CMV-antibody. (EVR: 80%, Cont: 87%)
*Conclusions: EVR as immunosuppressive induction therapy could delay the primary CMV infection after discontinuation of prophylactic VGCV and suppress the development of CMV disease.
To cite this abstract in AMA style:
Harada S, Sasaki H, Takada Y, Takamoto D, Harada H, Hirano T, Tanaka H. Suppressive Effect of De Novo Everolimus on Primary Cytomegalovirus Infection in Kidney Transplant Recipient [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/suppressive-effect-of-de-novo-everolimus-on-primary-cytomegalovirus-infection-in-kidney-transplant-recipient/. Accessed February 17, 2026.« Back to 2022 American Transplant Congress