Superior Renal Function with CNI-Free Everolimus over Standard CNI-Based Regimen: 18 Months Data from the Randomized, Multi-Center MANDELA Trial in De Novo Heart Transplant Recipients
1Mandela Study Group, Germany
2Novartis, Pharma, Germany.
Meeting: 2018 American Transplant Congress
Abstract number: 602
Keywords: Heart/lung transplantation, Immunosuppression, Renal function
Session Information
Session Name: Concurrent Session: Late Breaking
Session Type: Concurrent Session
Date: Tuesday, June 5, 2018
Session Time: 4:30pm-6:00pm
 Presentation Time: 5:18pm-5:30pm
Presentation Time: 5:18pm-5:30pm
Location: Room 3AB
Purpose: The MANDELA study (NCT00862979) was designed to assess the benefit on renal function of either CNI-free or CNI-minimized EVR-based regimen after early conversion of de novo heart transplant recipients (HTxR).
Methods: MANDELA is a multi-center, randomized, controlled, open-label, 12 month study. In total 232 de novo HTxR were enrolled 3 months post Tx, of whom 162 could be randomized (1:1) to receive either EVR (C0-h 5-10ng/mL) with reduced CNI (TAC C0-h 3-8ng/mL or CsA C0-h 50-150ng/mL) and steroids (≤0.3mg/kg) or EVR (C0-h 5-10ng/mL) with mycophenolic acid (EC-MPS max. 2880mg/day or MMF max. 3g/day) and steroids (≤0.3mg/kg). The primary objective was assessment of eGFR (MDRD) 12 months after randomization for superiority in CNI-free over CNI-reduced everolimus group. Key secondary objectives included efficacy (composite of BPAR ISHLT 1990 grade ≥3A / ISHLT 2004 grade ≥2R, graft loss / re-transplant, death or loss to follow-up) and assessment of safety profiles including infections.
Results: Primary endpoint for superior renal function in CNI-free EVR arm was met with high significance with a difference of +11.2 ml/min in favor of CNI-free EVR arm vs CNI-reduced group (p<0.0001) [cGFR (ml/min) MDRD; LS-mean ANCOVA with LOCF] (Fig.1: course of mean±SD cGFR; MDRD). Per protocol analysis showed a difference of +19.0 ml/min in favor of CNI-free EVR arm (p<0.0001) [cGFR (ml/min) from MDRD formula; LS-mean from ANCOVA model]. 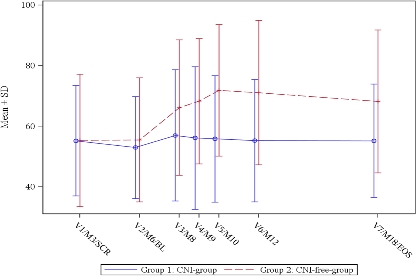
Rate of MACE and BPAR was in line with international standards and the safety profile according to the patient population and drugs investigated herein. Data from full analysis will be available for presentation at ATC2018 meeting.
Conclusion: The MANDELA study showed that improved renal function can be achieved by early conversion to an everolimus-based CNI-free regimen in HTxR without compromising safety and efficacy.
CITATION INFORMATION: Barten M. J., Hirt S. W., Garbade J., Bara C., Doesch A., Knosalla C., Grinninger C., Stypmann J., Sieder C., Junge M., Schulz U. Superior Renal Function with CNI-Free Everolimus over Standard CNI-Based Regimen: 18 Months Data from the Randomized, Multi-Center MANDELA Trial in De Novo Heart Transplant Recipients Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Barten MJ, Hirt SW, Garbade J, Bara C, Doesch A, Knosalla C, Grinninger C, Stypmann J, Sieder C, Junge M, Schulz U. Superior Renal Function with CNI-Free Everolimus over Standard CNI-Based Regimen: 18 Months Data from the Randomized, Multi-Center MANDELA Trial in De Novo Heart Transplant Recipients [abstract]. https://atcmeetingabstracts.com/abstract/superior-renal-function-with-cni-free-everolimus-over-standard-cni-based-regimen-18-months-data-from-the-randomized-multi-center-mandela-trial-in-de-novo-heart-transplant-recipients/. Accessed February 19, 2026.« Back to 2018 American Transplant Congress
