Six-Month Antibody Kinetics and Durability After Three Doses of SARS-CoV-2 Vaccine in Solid Organ Transplant Recipients
A. Abedon1, J. Alejo1, T. Chiang1, J. Mitchell1, J. Kim1, L. Thomas1, R. Avery1, A. Tobian1, A. Massie1, M. Levan1, D. Warren1, J. Garonzik-Wang2, D. Segev1, W. Werbel1
1Johns Hopkins, Baltimore, MD, 2University of Wisconsin, Madison, WI
Meeting: 2022 American Transplant Congress
Abstract number: 1639
Keywords: Antibodies, COVID-19, Vaccination
Topic: Clinical Science » Infection Disease » 24 - All Infections (Excluding Kidney & Viral Hepatitis)
Session Information
Session Name: All Infections (Excluding Kidney & Viral Hepatitis) IV
Session Type: Poster Abstract
Date: Tuesday, June 7, 2022
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
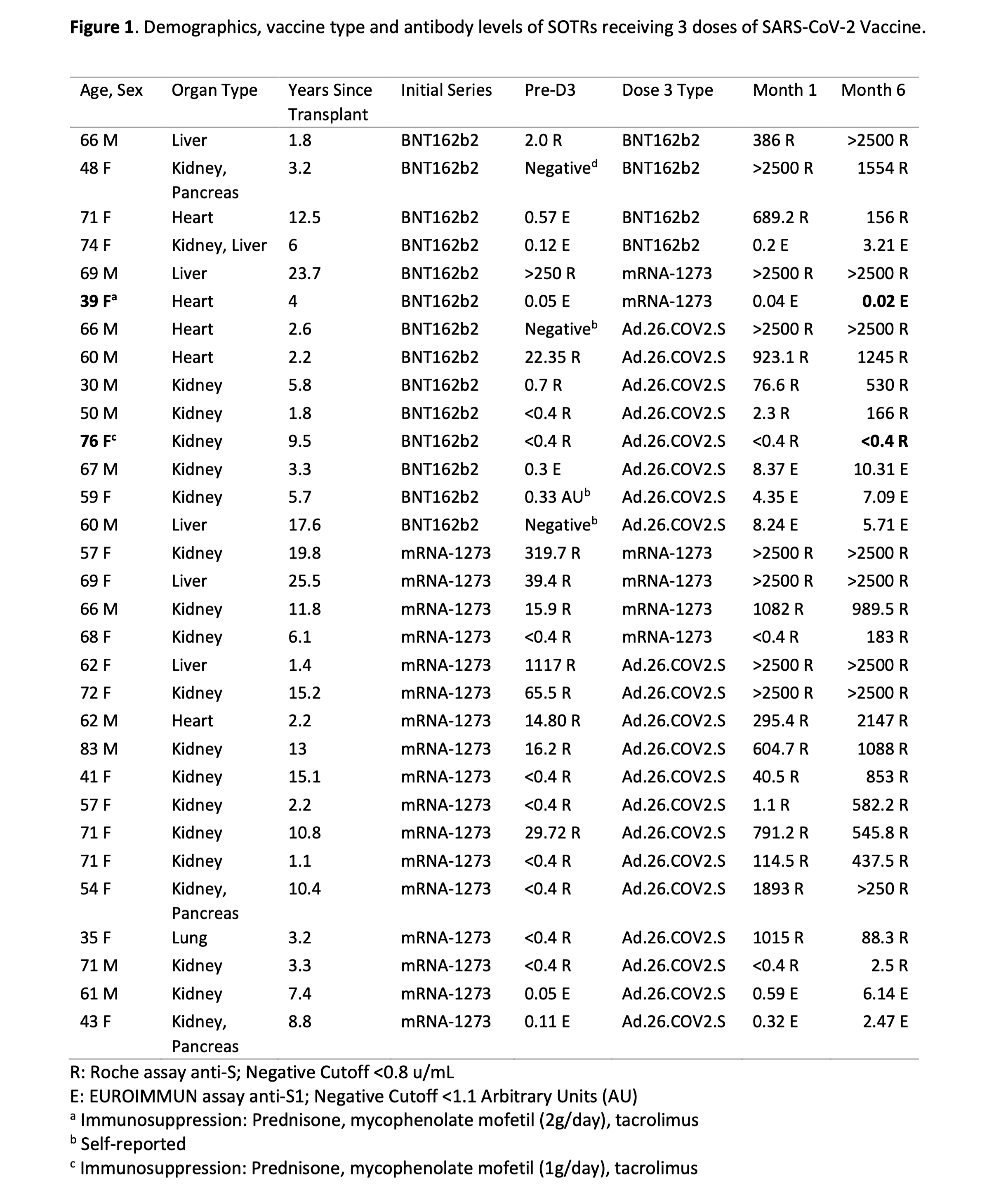
*Purpose: Some solid-organ transplant recipients (SOTRs) with low or negative antibody levels after a 2-dose mRNA vaccine series against SARS-CoV-2 experience boosting after a third dose (D3), but long-term antibody durability after D3 is unknown. We describe six-month SARS-CoV-2 antibody kinetics and durability in 31 SOTRs who received D3.
*Methods: 31 SOTRs without prior COVID-19 were identified within our national observational study. Serologic testing was performed a median of 30 (IQR 27-40) days after D3 and repeated at a median of 166 (148-184) days after D3. Semiquantitative anti-spike serologic testing using the Roche Elecsys anti-S enzyme immunoassay (EIA) or EUROIMMUN anti-S1 EIA was performed.
*Results: Over 6 months of follow-up, antibody levels increased in 16/27(59%), remained stable in 6/27(22%) (one negative, the others above the assay limit), and decreased in 5/27(19%). One-month post-D3, 24/31(77%) were seropositive and 7/31(23%) were seronegative. Six-months post-D3, 29/31(94%) were seropositive and 2/31(6%) remained seronegative. Both nonresponders received the BNT-162b2 primary series; one received Ad.26.CoV2.S and the other mRNA-1273 for D3. This difference in seroconversion after D3 was not statistically significant (Fisher exact = 0.49, between primary series). There were no reported cases of COVID-19 during the study period.
*Conclusions: We observed a very high rate of seroconversion after D3 in SOTRs, with marked heterogeneity in timing and strength of response depending on baseline antibody level and vaccine platform received. These results are encouraging evidence for the durable immunogenicity of additional vaccine doses in most SOTRs, and demonstrate the need for dedicated analysis of heterologous boosting strategies.
To cite this abstract in AMA style:
Abedon A, Alejo J, Chiang T, Mitchell J, Kim J, Thomas L, Avery R, Tobian A, Massie A, Levan M, Warren D, Garonzik-Wang J, Segev D, Werbel W. Six-Month Antibody Kinetics and Durability After Three Doses of SARS-CoV-2 Vaccine in Solid Organ Transplant Recipients [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/six-month-antibody-kinetics-and-durability-after-three-doses-of-sars-cov-2-vaccine-in-solid-organ-transplant-recipients/. Accessed February 17, 2026.« Back to 2022 American Transplant Congress