Single-Dose Basiliximab Induction Therapy in Low-Immunologic Risk Kidney Transplant Recipients
Pharmacy, Nebraska Medicine, Omaha, NE
Meeting: 2021 American Transplant Congress
Abstract number: 280
Keywords: Induction therapy, Kidney transplantation, Rejection, Simulect
Topic: Clinical Science » Kidney » Kidney Immunosuppression: Induction Therapy
Session Information
Session Name: Kidney Immunosuppression
Session Type: Rapid Fire Oral Abstract
Date: Monday, June 7, 2021
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:35pm-6:40pm
Presentation Time: 6:35pm-6:40pm
Location: Virtual
*Purpose: The purpose of this study is to examine the outcomes associated with single-dose basiliximab in low-immunologic risk kidney transplant recipients following a revision to the kidney transplant induction protocol at our institution.
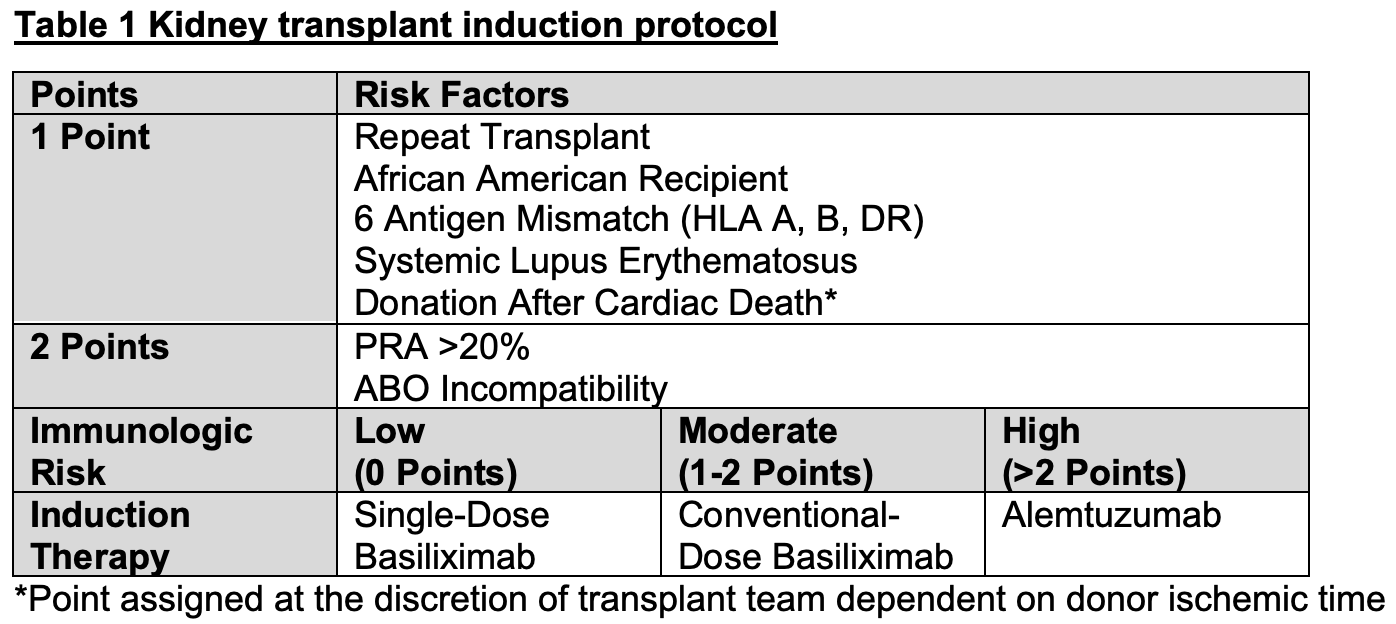
*Methods: A retrospective, single-center, observational chart review was conducted on kidney transplant recipients ≥19 years old who received single-dose basiliximab induction therapy from June 2019 to May 2020 based on the institution’s kidney transplant induction protocol (Table 1). All patients received a standard initial steroid taper and three-drug maintenance immunosuppression with tacrolimus, mycophenolate, and prednisone. Patients were excluded if they received lytic induction after receiving basiliximab or experienced graft loss prior to POD4. Patients who received single-dose basiliximab were assessed for incidence of biopsy proven acute cellular rejection (BPAR), antibody mediated rejection (AMR), graft loss, death, BK viremia, and cytomegalovirus (CMV) infection. Data on outcomes were collected for at least 6 months up to 12 months following transplantation.
*Results: There were 57 patients who received single-dose basiliximab during the review period. The incidence of BPAR was 3.5% with one patient experiencing Banff Grade III rejection resulting in graft loss. The incidence of AMR was 1.7% with one patient experiencing graft loss. Overall graft survival at the end of the study period was 96.5% and patient survival was 100%. CMV infection was observed in 24.5% of patients and 22.8% of patients had BK viremia.
*Conclusions: In this retrospective review, we observed low rates of BPAR, AMR, graft loss, and death in our low immunologic-risk kidney transplant recipients who received single-dose basiliximab for induction therapy. Single-dose basiliximab appears to be a safe and effective induction therapy regimen in appropriately selected low-immunologic risk kidney transplant recipients. Further study is needed to confirm the findings in this descriptive review.
To cite this abstract in AMA style:
Hutchins A, Schoen J, McMullen JS. Single-Dose Basiliximab Induction Therapy in Low-Immunologic Risk Kidney Transplant Recipients [abstract]. Am J Transplant. 2021; 21 (suppl 3). https://atcmeetingabstracts.com/abstract/single-dose-basiliximab-induction-therapy-in-low-immunologic-risk-kidney-transplant-recipients/. Accessed February 14, 2026.« Back to 2021 American Transplant Congress