Single Dose Alemtuzemab Provides Comparable Rejection Free and Infection Free Survival Compared to Double Dose Alemtuzumab. In Comparison to Alemtuzemab, Basiliximab Has Superior Infection Free Graft Survival. Results from a Steroid Sparing Transplant Group
Department of Transplant Surgery, Royal Liverpool University Hospital, Liverpool, United Kingdom
Meeting: 2022 American Transplant Congress
Abstract number: 1382
Keywords: Cytomeglovirus, Kidney transplantation, Rejection
Topic: Clinical Science » Kidney » 37 - Kidney Immunosuppression: Induction Therapy
Session Information
Session Name: Kidney Immunosuppression: Induction Therapy
Session Type: Poster Abstract
Date: Monday, June 6, 2022
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
*Purpose: The present study investigated the short-term biopsy-proven acute rejection rates and viral infection rates amongst our protocol of single-dose Alemtuzemab (Cohort 1) versus double dose Alemtuzemab (Cohort 0) induction with steroid-sparing and compared the results from standard Baxilimab (Cohort 2) induction at 30 days, 6-month and 12-month post-transplant.
*Methods: Data of all adult renal transplant recipients ( N=466) transplanted between 01/2012 and 01/2020 was analysed retrospectively. ABO , HLA incompatible allografts & early graft losses due to technical failures were excluded from analysis. Patients with cRF >20%, 2-DR mismatch, DCD-transplant, received Alemtuzumab for induction (30 mg sc before reperfusion and 24 hours post-transplant surgery). Recipients >60 years old received a single dose of Alemtuzumab for induction (30 mg sc before reperfusion). All other patients received standard IV Basiliximab for induction with stand maintenance dual immunosuppression.
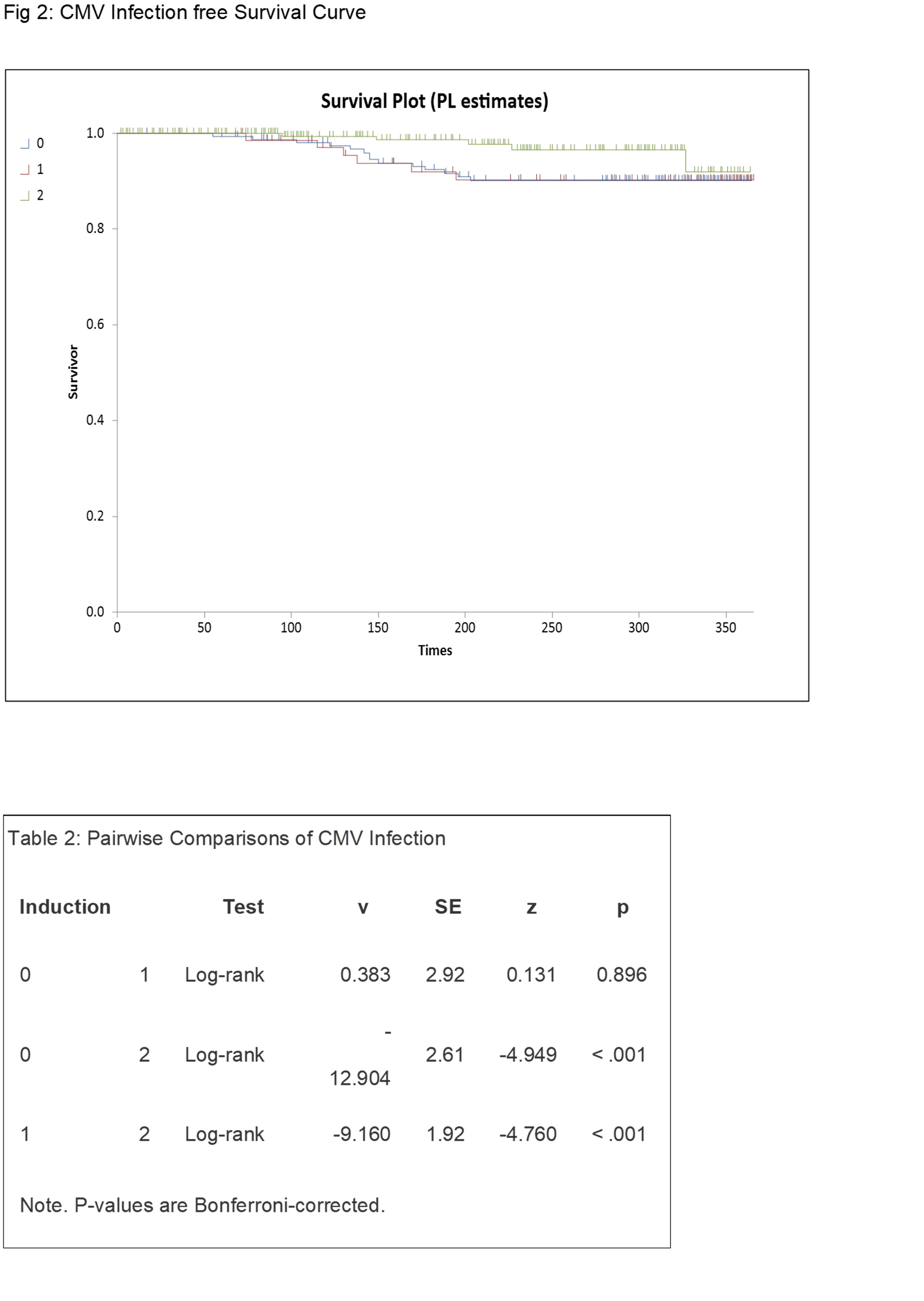
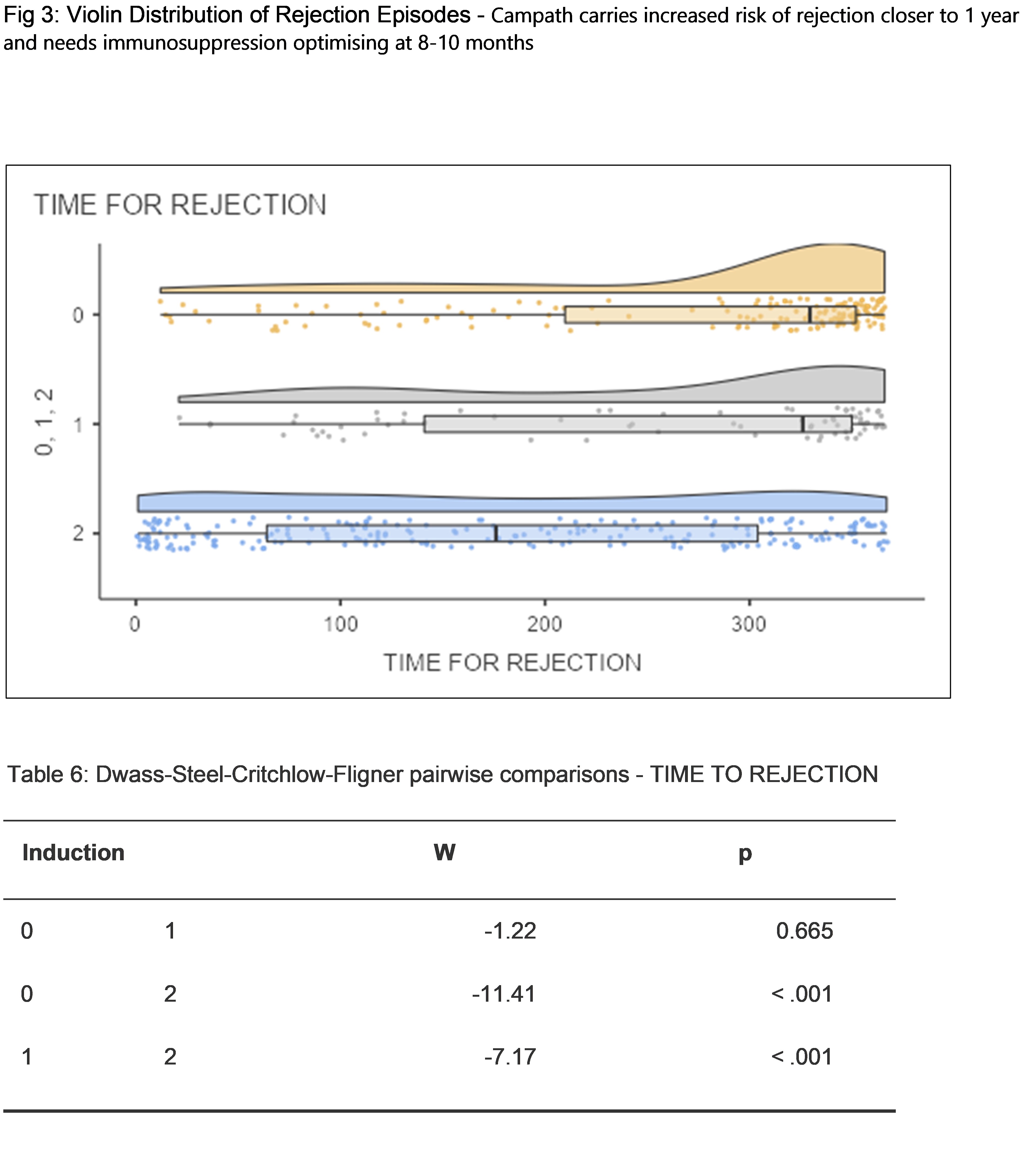
*Results: At 30 days, 06 months and 1-year post transplant the Acute Cellular Rejection rates were 1.2%, 1.7% and 5.2%; 6%, 5% and 10%; 7%, 8% and 11% for cohorts 0 , 1 and 2 respectively. At 30 days, 06 months and 1 year post transplant the CMV Infection rates were 0%, 0% and 0.4%; 7%, 8% and 1%; 9%, 11% and 1% for cohorts 0, 1 and 2 respectively.. At 30 days, 06 months and 1 year post transplant the BKV Infection rates were 1%, 2.5% and 1%; 15%, 15% and 6%, 4% and 7% for cohorts 0, 1 and 2 respectively.
*Conclusions: Our study provides non-inferior outcomes in biopsy-proven acute rejection rates and viral infection rates amongst our protocol of single-dose Alemtuzumab versus double dose Alemtuzumab induction with steroid-sparing. The outcomes of Alemtuzumab compared to the results from standard Basiliximab induction at thirty-day, six-month and twelve-month post-transplant show superior outcomes of Alemtuzumab in terms of rejection-free survival but a higher incidence of CMV infection rate
To cite this abstract in AMA style:
Sharma H, Odugoudar A, Devkaran B, Rao A, El-Bakry A, Gunawardhane T, Ridgway D, Howse M, Mehra S. Single Dose Alemtuzemab Provides Comparable Rejection Free and Infection Free Survival Compared to Double Dose Alemtuzumab. In Comparison to Alemtuzemab, Basiliximab Has Superior Infection Free Graft Survival. Results from a Steroid Sparing Transplant Group [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/single-dose-alemtuzemab-provides-comparable-rejection-free-and-infection-free-survival-compared-to-double-dose-alemtuzumab-in-comparison-to-alemtuzemab-basiliximab-has-superior-infection-free-graft/. Accessed March 7, 2026.« Back to 2022 American Transplant Congress