Semaglutide Tolerability and Efficacy in Patients with Chronic Kidney Disease Stage 4, 5 and End Stage Renal Disease
J. J. Long, A. Lemke, V. Sudhindran, N. Pencovici, A. Kukla, T. Diwan
Mayo Clinic, Rochester, MN
Meeting: 2022 American Transplant Congress
Abstract number: 790
Keywords: Kidney, Metabolic disease, Obesity
Topic: Clinical Science » Kidney » 35 - Kidney: Cardiovascular and Metabolic Complications
Session Information
Session Name: Kidney: Cardiovascular and Metabolic Complications
Session Type: Poster Abstract
Date: Saturday, June 4, 2022
Session Time: 5:30pm-7:00pm
 Presentation Time: 5:30pm-7:00pm
Presentation Time: 5:30pm-7:00pm
Location: Hynes Halls C & D
*Purpose: Obesity is often a barrier to kidney transplantation (KT) due to increased perioperative risks and worse outcomes with many transplant centers setting BMI cut offs in order to be active on the waitlist. Semaglutide, a GLP-1 agonist, has recently been shown to be an effective weight loss and diabetes treatment though outcomes in the setting of CKD (chronic kidney disease) and ESRD (End Stage Renal Disease) are unknown. The purpose of this study is to evaluate the tolerability and efficacy of semaglutide among patients with CKD and ESRD.
*Methods: This retrospective investigation included patients with CKD Stage 4, Stage 5, or ESRD from three transplant centers within one health system. Patients were identified by diagnosis code and semaglutide orders in the medical record Jan 2013 to Nov 2021. Investigators verified CKD diagnosis and manually extracted data of interest. Data was analyzed using Fischer’s exact test, paired T-test, and Wilcoxon signed rank test.
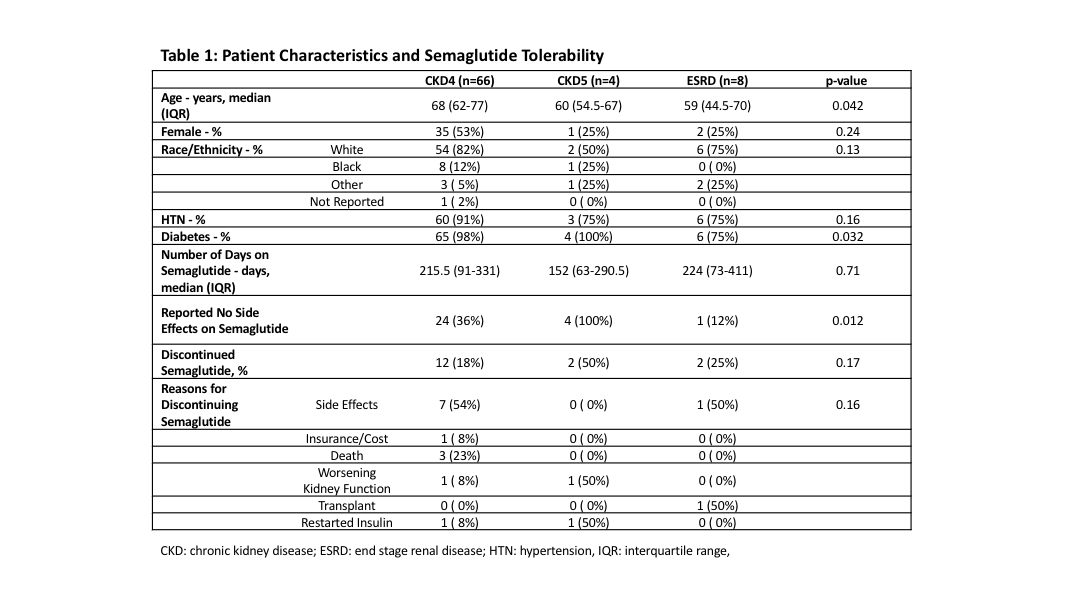
*Results: We identified 78 patients with CKD 4, CKD 5 or ESRD. The average starting dose of semaglutide was 3mg for oral and 0.3mg for injectable. The median time that patients were on semaglutide was 215.5 (IQR 91-331) days for CKD 4 patients, 152 (IQR: 63-290.5) for CKD5 patients, and 224 days (IQR 73-411, p=0.71) for ESRD patients. Of patients who discontinued semaglutide, side effects were the most common reason in 54% CKD 4, 0% CKD 5, and 50% ESRD patients (Table 1). The majority of side effects reported were GI related such as nausea, vomiting, and stomach pain; other side effects included weakness, syncope, and mood swings. However, 37% of patients reported no side effects while on semaglutide. After starting semaglutide, patients had a significant decrease in weight from 108±24.5 to 104±29.4kg (p<0.001) and HbA1c from 7.76±1.46 to 6.95±1.51% (p<0.001).
*Conclusions: This is the first study to demonstrate the use of semaglutide in patients with renal disease. Semaglutide can be safely used in this patient population with modest weight loss and improvement in diabetic parameters. However, more studies in larger populations are needed to better determine the risks and benefits and guide appropriate use of this medication, either independently or in conjunction with bariatric surgery.
To cite this abstract in AMA style:
Long JJ, Lemke A, Sudhindran V, Pencovici N, Kukla A, Diwan T. Semaglutide Tolerability and Efficacy in Patients with Chronic Kidney Disease Stage 4, 5 and End Stage Renal Disease [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/semaglutide-tolerability-and-efficacy-in-patients-with-chronic-kidney-disease-stage-4-5-and-end-stage-renal-disease/. Accessed March 9, 2026.« Back to 2022 American Transplant Congress