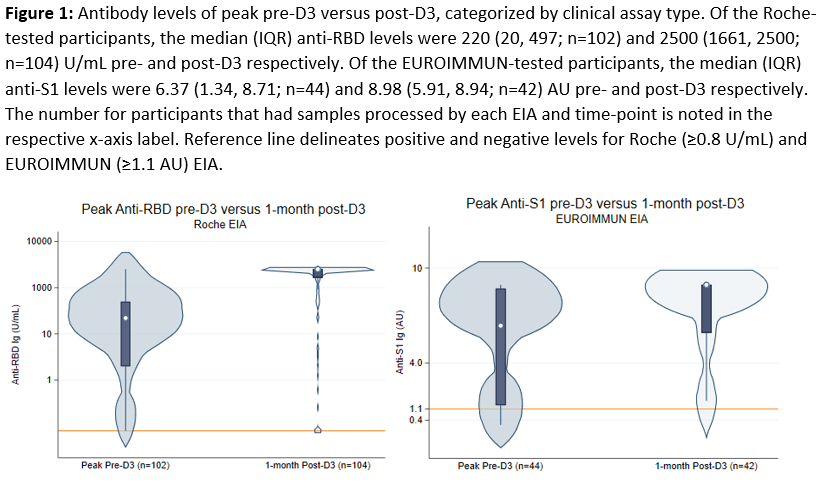
Sars-CoV-2 Antibody Response Before and After Third Dose of Homologous mRNA Vaccine in Liver Transplant Recipients
A. T. Strauss1, A. Chang1, J. Alejo1, T. Chiang1, L. Zeiser1, N. Fortune1, B. Boyarsky1, R. Avery2, A. A. Tobian1, M. Levan1, D. S. Warren1, A. Massie3, J. Garonzik-Wang1, D. Segev1, W. Werbel1
1Johns Hopkins University, Baltimore, MD, 2Johns Hopkins, Baltimore, MD, 3Johns Hopkins School of Medicine, Baltimore, MD
Meeting: 2022 American Transplant Congress
Abstract number: 1629
Topic: Clinical Science » Infection Disease » 24 - All Infections (Excluding Kidney & Viral Hepatitis)
Session Information
Session Name: All Infections (Excluding Kidney & Viral Hepatitis) IV
Session Type: Poster Abstract
Date: Tuesday, June 7, 2022
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
*Purpose: Liver transplant (LT) recipients have a decreased response to 2 doses of SARS-CoV-2 vaccine compared to the general population, so we aimed to understand response to a third dose to inform vaccination strategies.
*Methods: LT recipients in our observational cohort who received 3 homologous mRNA vaccines and available antibody levels pre- and post-dose 3 (D3) were identified. Those who reported a prior COVID-19 diagnosis or used belatacept were excluded. The peak anti-spike antibody level collected between the second (D2) and third dose (D3), was compared to the antibody level at 1 month post-D3. Samples were tested with Roche Elecsys Anti-Sars-CoV-2 enzyme immunoassay (EIA) (positive ≥0.8 U/mL) or EUROIMMUN EIA (positive ≥1.1 AU).
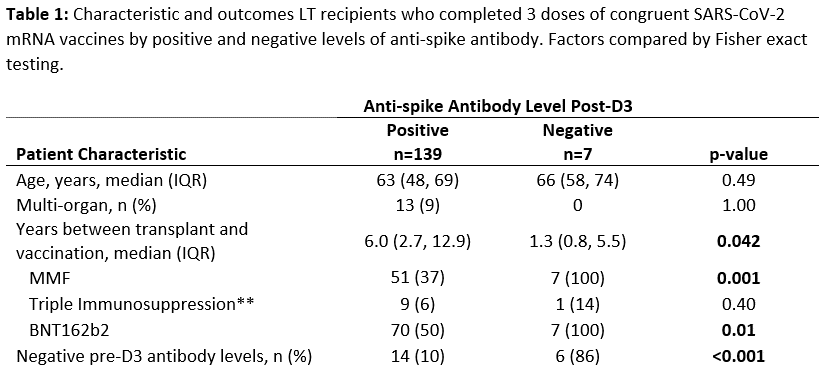
*Results: 146 participants completed 3 homologous doses of BNT162b2 (53%) or mRNA-1273 (47%) vaccines between 5/15/2021 – 11/8/2021. The median (IQR) time of peak pre-D3 antibody collection was 89 (31, 104) days post-D2. The median time of 1-month post-D3 antibody collection was 30 (23, 33) days. The median time between D2 and D3 was 168 (149-188) days. Overall, 125/146 (86%) were seropositive pre-D3, and 139/146 (95%) were seropositive post-D3 (Figure 1). There were no seroreversions post D3, and among the 21 seronegative recipients pre-D3, 14 (67%) seroconverted post-D3. Risk factors significantly associated with persistent seronegativity post-D3 were less time since LT (1.3 vs 6 years, p=0.042), mycophenolate use (100% vs 37%, p=0.001), BNT162b2 series (100% vs 50%, p=0.01), and pre-D3 seronegative status (86% vs 10%, p<0.001).
*Conclusions: Most LT recipients have excellent responses to a third homologous mRNA vaccine dose, greater than that seen in other transplant recipients. Persons seronegative after D2, however, show weaker response and may remain at high risk for SARS-CoV-2 infection despite D3.
To cite this abstract in AMA style:
Strauss AT, Chang A, Alejo J, Chiang T, Zeiser L, Fortune N, Boyarsky B, Avery R, Tobian AA, Levan M, Warren DS, Massie A, Garonzik-Wang J, Segev D, Werbel W. Sars-CoV-2 Antibody Response Before and After Third Dose of Homologous mRNA Vaccine in Liver Transplant Recipients [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/sars-cov-2-antibody-response-before-and-after-third-dose-of-homologous-mrna-vaccine-in-liver-transplant-recipients/. Accessed March 1, 2026.« Back to 2022 American Transplant Congress