Safety and Efficacy of Low Dose Rabbit Antithymocyte Globulin in Pediatric Renal Transplant Recipients
1Pediatric Nephrology, Stanford University, Stanford, CA, 2Surgery - Multi-Organ Transplantation, Stanford University, Stanford, CA
Meeting: 2020 American Transplant Congress
Abstract number: C-006
Keywords: Induction therapy, Infection, Pediatric, Rejection
Session Information
Session Name: Poster Session C: Kidney Immunosuppression: Induction Therapy
Session Type: Poster Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:00pm
 Presentation Time: 3:30pm-4:00pm
Presentation Time: 3:30pm-4:00pm
Location: Virtual
*Purpose: Currently there is no consensus among pediatric kidney transplant centers regarding the use and regimen for immunosuppressive induction therapy.The safety and effectiveness of reduced Rabbit Antithymocyte Globulin (ATG) ≤ 3.5 mg/kg cumulative dosing as induction therapy in low risk pediatric kidney transplant recipients is unknown.
*Methods: Pediatric renal transplant recipients transplanted 1/1/2013-5/1/2018 were considered for inclusion. Recipients of deceased or living donor organs and with least 12-month follow-up were included. “High risk”was defined by a repeat transplant, preformed donor specific antibodies (DSAs), peak panel-reactive antibodies >20%, or African-American race. Maintenance immunosuppression protocol was tacrolimus and mycophenolate mofetil, steroid free unless high risk. Outcomes were de novo DSA (dnDSA) formation, graft survival , biopsy proved rejection (BPR) and EBV/CMV/BK viremia/infection during the first 12 months. DSAs were routinely screened at 3,6 and 12 months. Protocol biopsies were done at 6 and 12 months and graded with Banff criteria. Additional DSA testing and/or biopsies were done if there was a clinical concern. Subclinical/borderline findings were included with or without treatment. Group 1: low risk patients, ATG dose of ≤ 3.5 mg/kg, Group 2: low risk patients receiving dose of >3.5 mg/kg and Group 3: high risk patients.
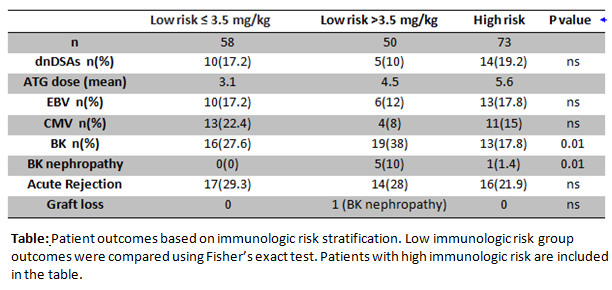
*Results: Results: A total of 181 patients met inclusion criteria. Age of patients was 11 years (11 mo-21 y),(median, range), 21% received a living donor transplant and 49% were female. Graft survival and dnDSA formation did not differ significantly between the three treatment groups. Graft loss at 12 months was a rare event with 99.5% graft survival and patient survival was 100%. Patients outcomes based on groups is shown below.
| “$$graphic_” |
*Conclusions: Reduced ATG dosing (≤ 3.5 mg/kg) when compared with higher dosing (>3.5 mg/kg) is safe and effective. Reduced ATG dose was associated with lower rates of BK viremia and BK nephropathy without increasing risk of dnDSA or BPR.
To cite this abstract in AMA style:
Sigurjonsdottir VK, Maestretti L, McGrath A, Gallo A, Concepcion W, Grimm P, Chaudhuri A. Safety and Efficacy of Low Dose Rabbit Antithymocyte Globulin in Pediatric Renal Transplant Recipients [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/safety-and-efficacy-of-low-dose-rabbit-antithymocyte-globulin-in-pediatric-renal-transplant-recipients/. Accessed March 12, 2026.« Back to 2020 American Transplant Congress