Safety and Efficacy of Anti Xa Inhibitors Post-Transplant with Concomitant Calcineurin Inhibitors
1Michigan Medicine, Ann Arbor, MI, 2Barnes-Jewish Hospital, St. Louis, MO, 3Veloxis Pharmaceuticals, Cary, NC
Meeting: 2019 American Transplant Congress
Abstract number: D211
Keywords: Anticoagulation, Heart/lung transplantation, Kidney/liver transplantation, Safety
Session Information
Session Name: Poster Session D: Non-Organ Specific: Pharmacogenomics / Pharmacokinetics
Session Type: Poster Session
Date: Tuesday, June 4, 2019
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall C & D
*Purpose: Despite demonstrated benefits of Anti-Xa Inhibitors (ATIs) in the general population, ATIs have not gained uniform acceptance in SOT due to lack of supportive data with concomitant calcineurin inhibitors (CNI) therapy given potential pharmacokinetic interactions and fluctuation in renal function. The purpose of this study is to evaluate safety and efficacy of ATIs in combination with CNIs in SOT recipients.
*Methods: This was a single-center, retrospective review of all SOT recipients including kidney, liver, pancreas, heart and lung conducted at Barnes-Jewish Hospital. All adult SOT recipients prescribed CNIs and ATI concurrently for at least 72 hours at any time point post-transplant between 01/01/2013—06/01/2017 were included for review. The primary outcome was the rate of total bleeding events in SOT recipients on concurrent CNIs and ATIs. Secondary outcomes included the rate of major and minor bleeding events, and rate of thrombotic events. Major bleed was defined as a clinically overt bleeding associated with 1 of the following: death, bleeding from a critical site (intracranial, intraspinal, intraocular, retroperitoneal, intramuscular, intra-articular, or pericardial), hemoglobin drop of ≥2 g/dL, or transfusion of ≥2 units of packed red blood cells (PRBCs). All other clinically significant bleeding not meeting the definition for major bleeding and led to interruption of ATI or dose modification for ≥ 24 h, surgical intervention, or transfusion of ≥ 1 unit of PRBC was a minor bleed.
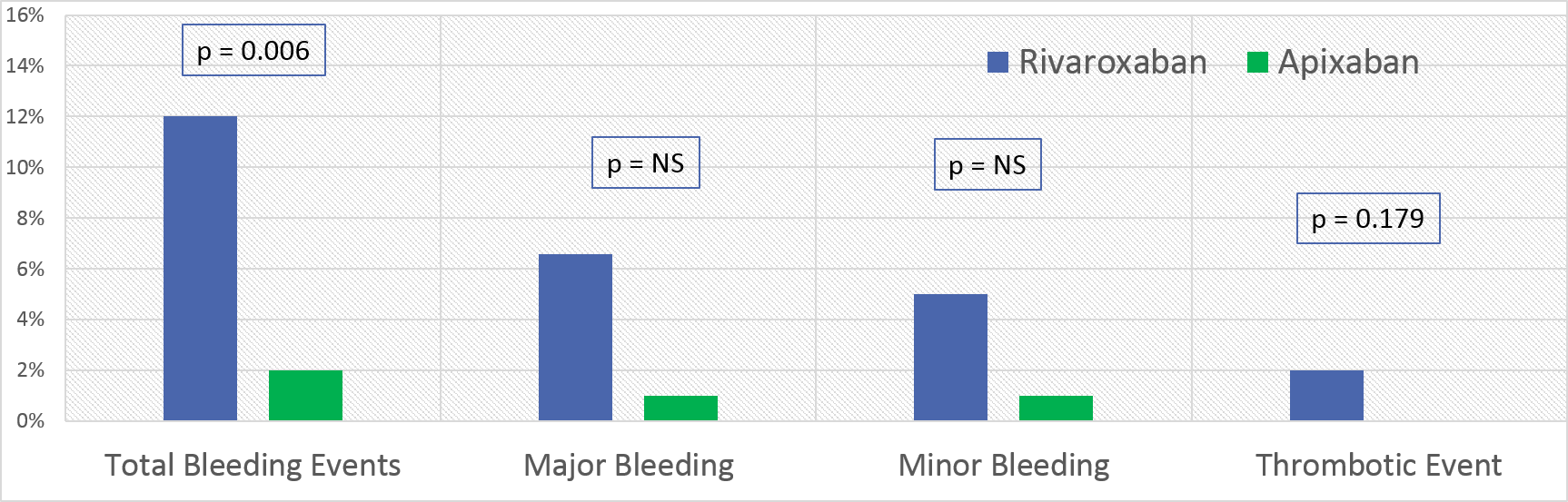
*Results: There were 172 SOT recipients who met criteria for inclusion with 70 receiving rivaroxaban and 102 receiving apixaban. Baseline characteristics were similar with the exception of variation between organ groups. Total bleeding rate was higher in rivaroxaban group, 9 (12%) vs 2 (2%), p = 0.006 (Figure 1). Median days to major bleeding events was longer in the rivaroxaban group vs apixaban group from ATI initiation (95 vs 306, p = 0.014). Thrombotic events were numerically similar in patients on rivaroxaban vs apixaban (2 vs 0, p=0.179). Estimated creatinine clearance at max serum creatinine, ml/min/m2 (median, IQR) was higher in the rivaroxaban group vs apixaban group (51.8 (34.3-69.9 vs 35.5 (23.3-47.9)), p = 0.004). At any time during ATI therapy, fewer patients on rivaroxaban were overdosed based on indication/renal function compared to apixaban (21.4% vs 33.3%, p <0.001).
*Conclusions: In this single center study of CNI treated SOT recipients, apixaban was associated with a lower rate of bleeding comparted to rivaroxaban
To cite this abstract in AMA style:
McMurry KA, Shuster J, Bain K, Horwedel T, Hartupee J. Safety and Efficacy of Anti Xa Inhibitors Post-Transplant with Concomitant Calcineurin Inhibitors [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/safety-and-efficacy-of-anti-xa-inhibitors-post-transplant-with-concomitant-calcineurin-inhibitors/. Accessed March 4, 2026.« Back to 2019 American Transplant Congress