Safety and Efficacy of 3-Month Ciprofloxacin for BK Prophylaxis in Kidney Transplantation
Houston Methodist Hospital, Houston, TX.
Meeting: 2015 American Transplant Congress
Abstract number: A19
Keywords: Infection, Polyma virus, Prophylaxis
Session Information
Session Name: Poster Session A: BK Virus Infection
Session Type: Poster Session
Date: Saturday, May 2, 2015
Session Time: 5:30pm-7:30pm
 Presentation Time: 5:30pm-7:30pm
Presentation Time: 5:30pm-7:30pm
Location: Exhibit Hall E
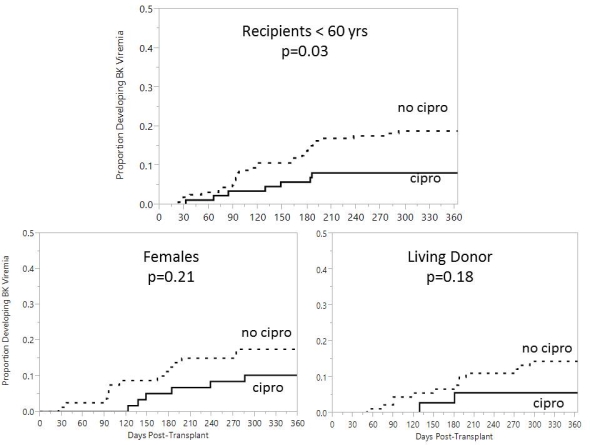
Flouroquinolones(FQ) have been shown to inhibit BK virus (BKV) in vitro, however clinical data remains mixed. We reviewed a pilot protocol of BK prophylaxis with ciprofloxacin (CIP) in a large cohort of kidney transplant recipients. From Mar. 2012 to Dec. 2012, 125 patients were discharged on CIP 500 mg/day for 3 months. Plasma BK PCRs were measured at months 1,3,6 and 12 post-transplant. All patients received antibody induction and tacrolimus, mycophenolate +/- prednisone. 232 patients transplanted from Jan. 2011 to Feb. 2012 served as controls (no-CIP). Baseline differences included fewer males (52% vs. 66%,p=0.01) more ATG use, (74% vs. 58%, p<0.01) and less prednisone use (85% vs. 91%, p=0.06) in the CIP group. No differences existed in tacrolimus levels or mycophenolate dosages. CIP-treated patients had a non-significantly lower rate of BKV at all time points, resulting in 30% lower rate 1 year (12% vs. 17.2%; p=0.18); Kaplan-Meier analysis revealed similar findings (Fig 1). No differences were seen in the rate of BK nephropathy (4.8% vs. 4.7%) or inital and peak PCRs. Subgroup analyses revealed a significantly lower rate of BKV among CIP patients who were younger (<60 year) and slight advantages in female and living donor recipients (Fig 2). No differences were seen in urinary tract infections or clostridium difficile, but CIP-patients had a lower rate of bacteremias and a higher rate of FQ-resistance gram-negative infections (Table 1). CIP prophylaxis resulted in a non-significant reduction in BKV. A secondary benefit in preventing bacteremias was seen, but at the expense of greater FQ resistance. A randomized controlled trial is currently underway (NCT01789203). 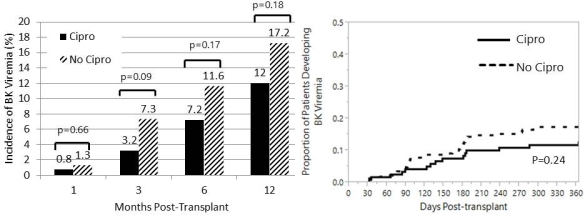
| Infection Type | CIP | No-CIP | P-value |
| Urinary tract | 25% | 38% | 0.12 |
| Bacteremia | 0 | 7% | <0.01 |
| C.Diff | 2.4% | 2.2% | 0.88 |
| FQ resistant infection | 65% | 36% | 0.04 |
To cite this abstract in AMA style:
Patel S, Knight R, Loucks J, Kuten S, Gaber A. Safety and Efficacy of 3-Month Ciprofloxacin for BK Prophylaxis in Kidney Transplantation [abstract]. Am J Transplant. 2015; 15 (suppl 3). https://atcmeetingabstracts.com/abstract/safety-and-efficacy-of-3-month-ciprofloxacin-for-bk-prophylaxis-in-kidney-transplantation/. Accessed March 7, 2026.« Back to 2015 American Transplant Congress
