Rituximab To Prevent Renal Allograft Rejection; a Randomized, Double-Blind, Placebo-Controlled Trial
Nephrology, Radboud University Nijmegen Medical Centre, Nijmegen, Netherlands
Pathology, Radboud University Nijmegen Medical Centre, Nijmegen, Netherlands
Meeting: 2013 American Transplant Congress
Abstract number: 266.1
Background
Anti-B-cell therapy could reduce the incidence of acute renal allograft rejection, especially in immunized patients. This study evaluated the efficacy and safety of rituximab added to standard immunosuppression.
Methods
We randomly assigned 280 adult renal transplant patients to receive a single dose of rituximab (375 mg/m2) or placebo as induction therapy during transplant surgery. Concomitant immunosuppressive therapy consisted of tacrolimus, mycophenolate mofetil, and steroids. Patients were stratified for PRA (panel reactive antibodies) and retransplantation. The primary endpoint was the incidence of biopsy proven acute rejection within 6 months after transplantation. Secondary endpoints included graft function, patient and graft survival, and the incidence of infections and malignancies at 24 months (data available for 180 patients).
Results
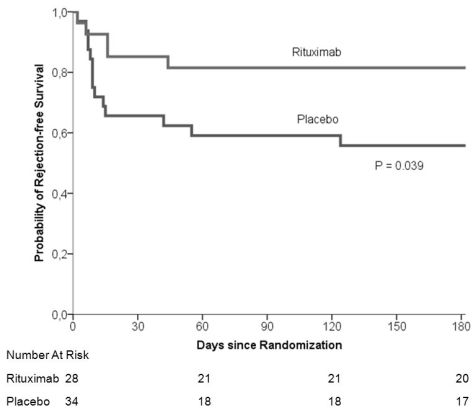
Biopsy proven acute rejection occurred in 22 of 138 rituximab treated patients (15.9%), compared to 31 of 142 placebo treated patients (21.8%, P=0.15). In immunized patients (PRA >6% and retransplants, n=62), rituximab significantly reduced the incidence of biopsy proven rejection (17.9% vs 41.1%; P=0.039, see Figure 1). There was no difference in graft function. At 6 months, leucopenia (<3.0 x 10E9/l) occurred more frequently in rituximab treated patients (6.5% vs 0%, P<0.01). There was no difference in the incidence of trombocytopenia or anemia. Within 24 months after transplantation, there was no difference in the incidence of infections and malignancies. At 24 months, graft and patient survival were comparable between both groups (88.7% vs 87.7%, P=0.93, and 92.3% vs 92.8%, P=0.87).
Conclusion
A single dose of rituximab added to standard immunosuppressive therapy reduces the incidence of biopsy proven renal allograft rejection in immunized patients. Treatment with rituximab appears to be safe, but it is associated with an increased incidence of leucopenia. (ClinicalTrials.gov number NCT00565331)
Figure 1. Kaplan–Meier Estimates of Rejection-free Survival in Immunized Patients.
Hilbrands, L.: Grant/Research Support, Roche. Wolf, J.: Grant/Research Support, Roche.
To cite this abstract in AMA style:
Hoogen Mvanden, Steenbergen E, Hoitsma A, Hilbrands L. Rituximab To Prevent Renal Allograft Rejection; a Randomized, Double-Blind, Placebo-Controlled Trial [abstract]. Am J Transplant. 2013; 13 (suppl 5). https://atcmeetingabstracts.com/abstract/rituximab-to-prevent-renal-allograft-rejection-a-randomized-double-blind-placebo-controlled-trial/. Accessed July 5, 2025.« Back to 2013 American Transplant Congress
