Results from the LIVE-C Free Trial
J. N. Fleming1, D. DuBay1, N. L. Jonassaint2, R. S. Satoskar3, K. D. Chavin4
1Medical University of South Carolina, Charleston, SC, 2University of Pittsburgh Medical Center, Pittsburgh, PA, 3Medstar Georgetown Medical Center, Washington, DC, 4University Hospital, Cleveland, OH
Meeting: 2019 American Transplant Congress
Abstract number: 591
Keywords: Hepatitis C, Liver transplantation
Session Information
Session Name: Concurrent Session: Non-Organ Specific: Viral Hepatitis
Session Type: Concurrent Session
Date: Tuesday, June 4, 2019
Session Time: 4:30pm-6:00pm
 Presentation Time: 5:42pm-5:54pm
Presentation Time: 5:42pm-5:54pm
Location: Room 309
*Purpose: Ledipasvir/sofosbuvir (LDV/SOF) in combination with ribavirin (RBV) has shown excellent results post-liver transplant. However, due to the poor tolerability of RBV, it is commonly questioned whether LDV/SOF monotherapy would be equally as efficacious. Additionally, a short course of LDV/SOF has shown preliminary feasibility immediately post-transplant, however may be challenging to replicate. We aimed to compare the efficacy and safety of LDV/SOF monotherapy with LDV/SOF+RBV in patients post-liver transplant, as well as determine the ability of LDV/SOF monotherapy for 8 weeks at preventing recurrence if given within 90 days post-transplant.
*Methods: This was a multicenter, randomized, open-label, phase IV study in patients post-liver transplant with genotype 1 or 4 HCV. Patients within 90 days of transplant (Early Cohort) were randomized to receive either 8 or 12 weeks of LDV/SOF monotherapy (target N = 60) and patients more than 90 days out from transplant (Late Cohort) were randomized to LDV/SOF monotherapy or LDV/SOF+RBV for 12 weeks (target N = 170).
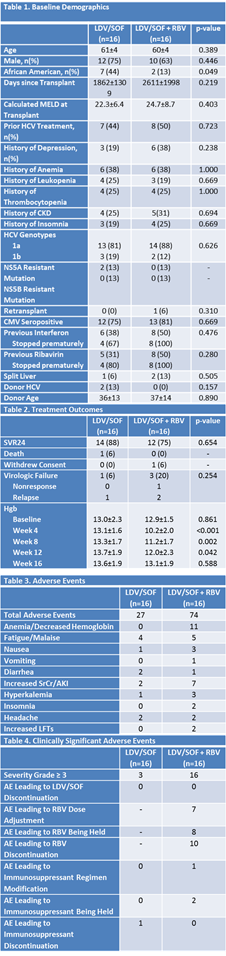
*Results: The Early Cohort was discontinued after 3 patients had been enrolled and the study was halted after 32 patients had been enrolled in the Late Cohort. One patient in the Early Cohort received 12 weeks of LDV/SOF and was included in the Late Cohort data. Two patients in the Early Cohort received 8 weeks of LDV/SOF, one of whom had virologic relapse with a newly detected NS5A mutation and one of whom died from a cause unrelated to treatment. Within the Late Cohort, there were no differences amongst baseline demographics between Late Cohort groups, although 2 patients in the LDV/SOF group had a mutation that predicted NS5A resistance, but were determined clinically insignificant by investigators (Table 1). Treatment outcomes were similar between groups, with 88% of the LDV/SOF cohort and 75% of the LDV/SOF+RBV cohort achieving SVR24 (Table 2). There was one death, unrelated to treatment, in the LDV/SOF cohort and one relapse. The relapse occurred in one of the two patients with a detected NS5A resistant mutation. In the LDV/SOF+RBV cohort, there was one patient that withdrew consent, one nonresponse, and two relapses. No patient that had virologic failure had a newly detected mutation predicting resistance. Treatment tolerability appeared to favor the LDV/SOF cohort over the LDV/SOF+RBV groups (Table 3 and 4).
*Conclusions: Due to changes in the national treatment atmosphere in the time it took to begin enrollment, there was a paucity of untreated HCV patients being transplanted. At any time post-liver transplant, ribavirin inclusion as part of the antiviral regimen leads to more overall and clinically significant adverse effects, and does not appear to improve treatment efficacy.
To cite this abstract in AMA style:
Fleming JN, DuBay D, Jonassaint NL, Satoskar RS, Chavin KD. Results from the LIVE-C Free Trial [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/results-from-the-live-c-free-trial/. Accessed February 22, 2026.« Back to 2019 American Transplant Congress