Renal Allograft Tolerance Can Be Achieved in Non-Human Primates Via Delayed Mixed-Hematopoietic Chimerism and Alefacept Treatment
Transplant Surgery, Massachusetts General Hospital, Boston, MA
Pathology, Massachusetts General Hospital, Boston, MA
Meeting: 2013 American Transplant Congress
Abstract number: 380
Renal allograft tolerance has been achieved in MHC-mismatched NHPs and patients via the mixed-chimerism approach. Activation/expansion of donor-reactive CD8+ effector memory T cells (TEMs) represents a major barrier to tolerance induction using our delayed mixed chimerism protocol. In this study, we tested whether, Alefacept, a fusion protein composed of IgG Fc portion and LFA-3, known to deplete selectively CD2highCD8+ TEMs could promote tolerance to kidney allografts in our model.
20 Cynomolgus monkeys underwent KTx with a triple drug immunosuppression(IS) (tacrolimus, mycophenolate mofetil and prednisone). Four months after KTx, recipients received a nonmyeloablative conditioning regimen with or without additional treatment against CD8 TEM and received BMT from the kidney donor. Then they were treated with a 1-month course of cyclosporine. Recipients were divided into 3 groups : Group A (control, n=5), B (anti-CD8 mAb, n= 12), C (LFA3-Ig, n=3).
In Group A, all recipients failed to develop chimerism and rejected allografts. In Group B, 10/12 developed chimerism and 6/10 survived long term without IS, 3 died due to EBV related lymphoma or BK virus infection and the last one and the two who failed chimerism developed acute rejection. In Group C, all recipients developed multilineage chimerism without infectious complication or lymphoma and survived long-term without IS. The Alefacept significantly delayed the expansion of CD2 high CD8+ TEM while it did not affect the recovery of CD8+ naive T cells. All recipients maintained stable normal renal function (creatinine <2.0mg/dl) for more than 300 days.
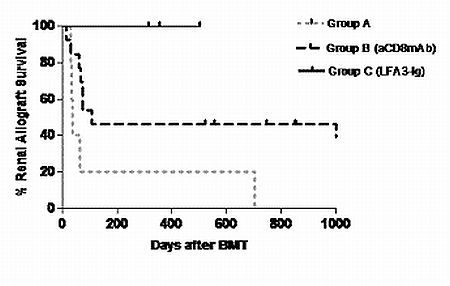
Alefacept enhanced induction of chimerism and promoted renal allograft tolerance. Targeting of CD2high T cells represents a promising approach to overcome the expansion/activation of donor-reactive TEM thus promoting tolerance induction to allografts in NHPs.
To cite this abstract in AMA style:
Lee S, Yamada Y, Boskovic S, Atif M, Tonsho M, Nadazdin O, Smith R, Colvin R, Allan J, Madsen J, Cosimi A, Benichou G, Kawai T. Renal Allograft Tolerance Can Be Achieved in Non-Human Primates Via Delayed Mixed-Hematopoietic Chimerism and Alefacept Treatment [abstract]. Am J Transplant. 2013; 13 (suppl 5). https://atcmeetingabstracts.com/abstract/renal-allograft-tolerance-can-be-achieved-in-non-human-primates-via-delayed-mixed-hematopoietic-chimerism-and-alefacept-treatment/. Accessed July 18, 2025.« Back to 2013 American Transplant Congress
