Reduced Dose Valganciclovir is Effective for CMV Prevention without Excess Breakthrough or Resistance in Kidney Transplantation
1Pharmacy, University of Colorado Hospital, Aurora, CO, 2Medicine, University of Colorado, Aurora, CO
Meeting: 2020 American Transplant Congress
Abstract number: 380
Keywords: Cytomeglovirus, Infection, Kidney transplantation
Session Information
Session Name: CMV and other Herpes Viruses
Session Type: Oral Abstract Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:45pm
 Presentation Time: 4:03pm-4:15pm
Presentation Time: 4:03pm-4:15pm
Location: Virtual
*Purpose: Reduced dose valganciclovir (VGC) (450 mg daily) may be a useful alternative to full dose VGC for cytomegalovirus (CMV) prophylaxis due to drug cost and toxicity, but little data is known about the risk of CMV breakthrough or drug resistance with this dosing strategy. The purpose of this study was to evaluate the incidence, timeline to development, and outcomes of CMV disease in a large cohort for renal recipients on reduced dose VGC prophylaxis.
*Methods: 1060 patients who received a kidney or kidney-pancreas transplant between 01/2012 and 12/2018 were included in this single center retrospective analysis. Primary immunosuppression was tacrolimus, mycophenolate, and prednisone along with thymoglobulin (r-ATG) induction. Patients with a CMV Donor(D)+/CMV Recipient(R)- serostatus received VGC 450 mg daily for 6 months, CMV R+ patients who received r-ATG induction were given VGC 450 mg daily for 3 months, and D-/R- patients did not receive CMV prophylaxis. The primary endpoint was the 12 month incidence of CMV disease, defined as CMV DNAemia along with systemic signs or symptoms. Secondary outcomes included rate of CMV breakthrough while on prophylaxis, recurrent CMV disease, and CMV resistance.
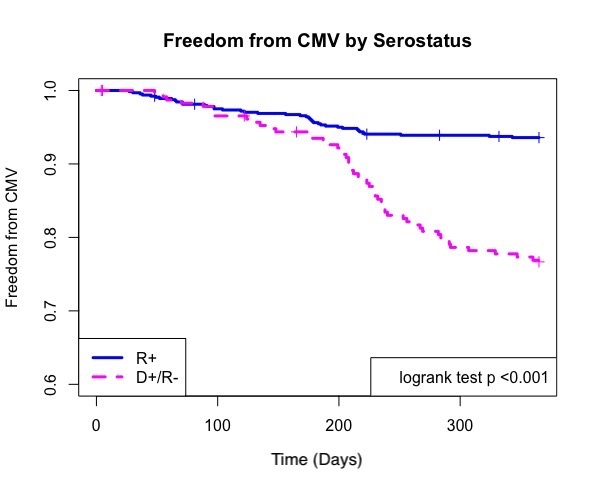
*Results: Overall 1 year CMV disease rate was 9.2% with risk stratification distributions reported in table 1.
| CMV Serostatus | N | + CMV Disease |
| D+/ R- | 233 | 64 (27.5%) |
| R+ | 641 | 44 (6.9%) |
| D-/ R- | 186 | 2 (1.1%) |
Risk factors for development of CMV included CMV mismatch (OR=4.4 , p <0.001) and any rejection episode (OR=1.9 , p=0.04 ), both of which were also risks for breakthrough CMV (mismatch OR=9.2 , p<0.001; any rejection OR=3.0, p=0.04). Recurrent disease occurred in 11.8% of patients who developed CMV. Serostatus did not predict recurrent disease. Breakthrough disease occurred in 6.4% of D+/R- and 0.8% of R+ patients. CMV resistance was rare in those with CMV disease and occurred in one patient (0.9%).
*Conclusions: Reduced dose VGC provided comparable rates of CMV disease as reported in the literature with full dose (900 mg daily) and was not associated with excess breatkthrough or resistance. Further monitoring after completion of VGC prophylaxis in CMV mismatch patients should be considered to reduce risk of late disease.
To cite this abstract in AMA style:
Luu M, Klem P, Crowther B, Abidi M, Miller M, Schoeppler K, Davis S. Reduced Dose Valganciclovir is Effective for CMV Prevention without Excess Breakthrough or Resistance in Kidney Transplantation [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/reduced-dose-valganciclovir-is-effective-for-cmv-prevention-without-excess-breakthrough-or-resistance-in-kidney-transplantation/. Accessed February 25, 2026.« Back to 2020 American Transplant Congress