Real Time Assessment of Kidney Transplant Indication Biopsies by Microarrays: First Results of the INTERCOMEX Study.
1University of Alberta, Edmonton, Canada
2ATAGC, Edmonton, Canada.
Meeting: 2016 American Transplant Congress
Abstract number: D305
Keywords: Antibodies, Biopsy, Kidney, Rejection
Session Information
Session Name: Poster Session D: Late Breaking
Session Type: Poster Session
Date: Tuesday, June 14, 2016
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Halls C&D
Oncology uses central molecular tissue assessment (MTA) to guide management but the potential of this approach in transplantation is not known. We undertook a prospective trial of real-time central MTA in 526 indication biopsies performed between 2014-15 in North American and European centers (Clinicaltrials.gov NCT01299168). Using modified Affymetrix microarray technology, the molecular microscope diagnostic system (MMDx) assessed T cell-mediated rejection (TCMR), antibody-mediated rejection (ABMR), acute kidney injury (AKI), and irreversible injury (atrophy-fibrosis). Biopsies stabilized in RNAlater were transmitted at room temperature to the central laboratory and processed (processing time 30 hours) to generate an automated report independent of histology and DSA. We compared the results to local histology and sought feedback from clinicians. Biopsies were between 0 and 27 years post-transplant. By Jan 2016, 501 biopsies had complete data for analysis. Rejection diagnoses by histology were ABMR (18%), TCMR (6%), and mixed (1%). As in previous studies, after 10 years post-transplant TCMR was extremely rare but ABMR was common. A molecular classifier for non-adherence was positive in 22 biopsies (4%), usually between 1-5 years, with rejection: 13 TCMR, 3 ABMR, 5 mixed. MMDx diagnoses correlated strongly with histologic diagnoses (Table 1): The molecular phenotype agreed with the conventional phenotype of TCMR (accuracy 90%) and ABMR (accuracy 78%), but with many disagreements. Comparison of local conventional to central MMDx testing reveals unexpected variation in histology interpretation among centers nominally using Banff, and differences within centers between pathologist and clinician interpretation. In 149 feedback forms, clinicians indicated that 128 (86%) agreed with clinical judgement, despite disagreement with histology; 84% indicated that MMDx invited more confidence for management decisions and that in 1/3 of biopsies MMDx report would alter therapy and/or investigations. We conclude that real-time MTA offers a new dimension in adding precision to biopsy interpretation. Clinicaltrials.govNCT#01299168 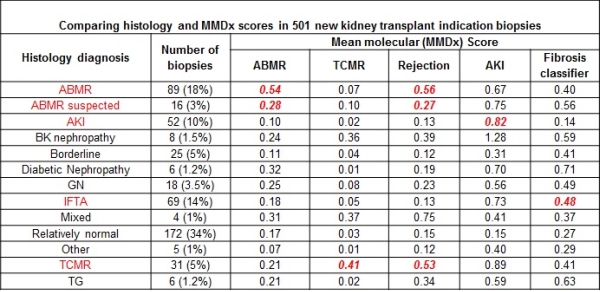
CITATION INFORMATION: Halloran P, Reeve J, INTERCOMEX Study Group Real Time Assessment of Kidney Transplant Indication Biopsies by Microarrays: First Results of the INTERCOMEX Study. Am J Transplant. 2016;16 (suppl 3).
To cite this abstract in AMA style:
Halloran P, Reeve J, Group INTERCOMEXStudy. Real Time Assessment of Kidney Transplant Indication Biopsies by Microarrays: First Results of the INTERCOMEX Study. [abstract]. Am J Transplant. 2016; 16 (suppl 3). https://atcmeetingabstracts.com/abstract/real-time-assessment-of-kidney-transplant-indication-biopsies-by-microarrays-first-results-of-the-intercomex-study/. Accessed February 25, 2026.« Back to 2016 American Transplant Congress
