Racial Differences in Tacrolimus Xr Dosing in De Novo Kidney Transplant Recipients
Medical University of South Carolina, Charleston, SC
Meeting: 2021 American Transplant Congress
Abstract number: 1273
Keywords: African-American, Immunosuppression
Topic: Clinical Science » Organ Inclusive » Non-Organ Specific: Disparities to Outcome and Access to Healthcare
Session Information
Session Name: Non-Organ Specific: Disparities to Outcome and Access to Healthcare
Session Type: Poster Abstract
Session Date & Time: None. Available on demand.
Location: Virtual
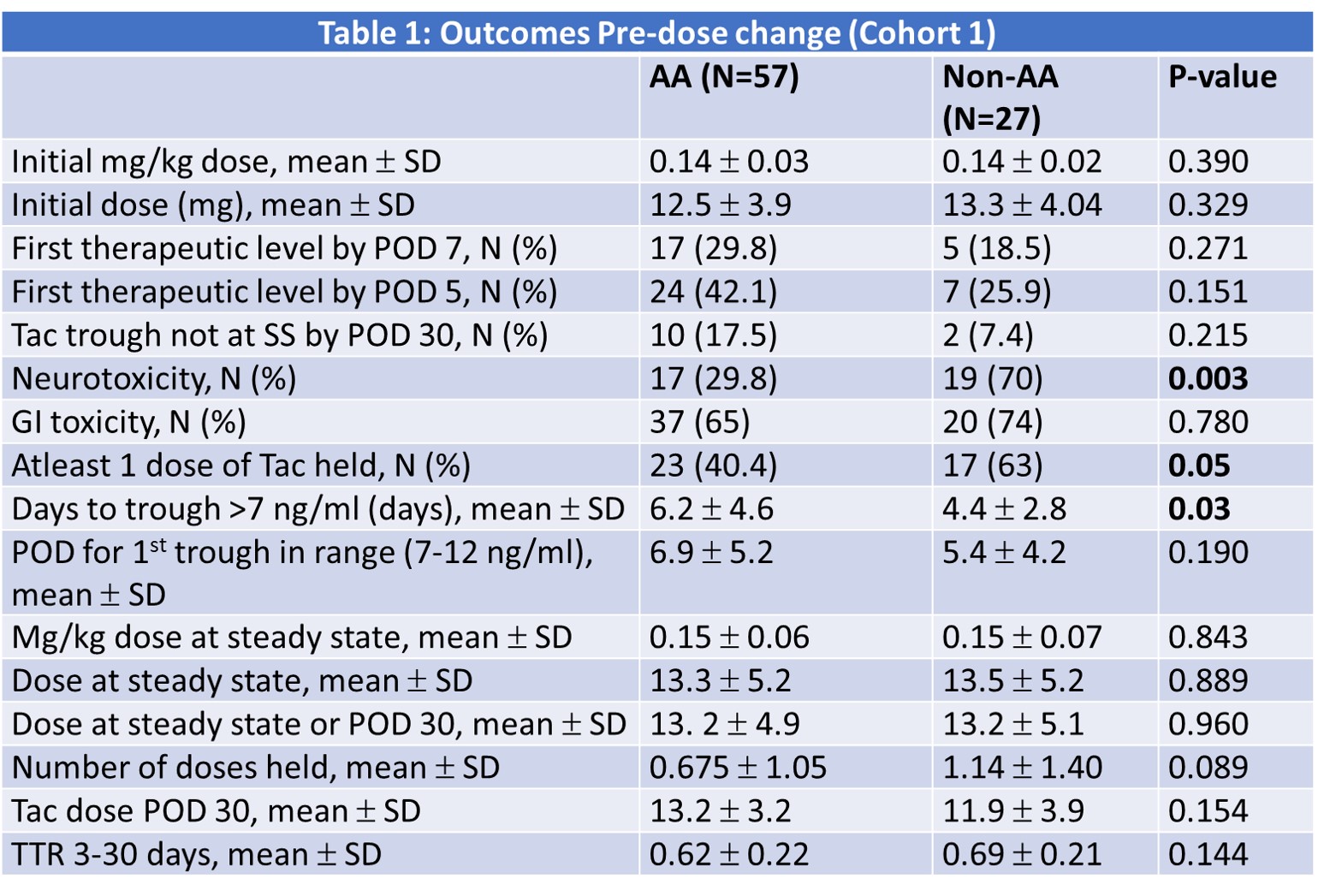
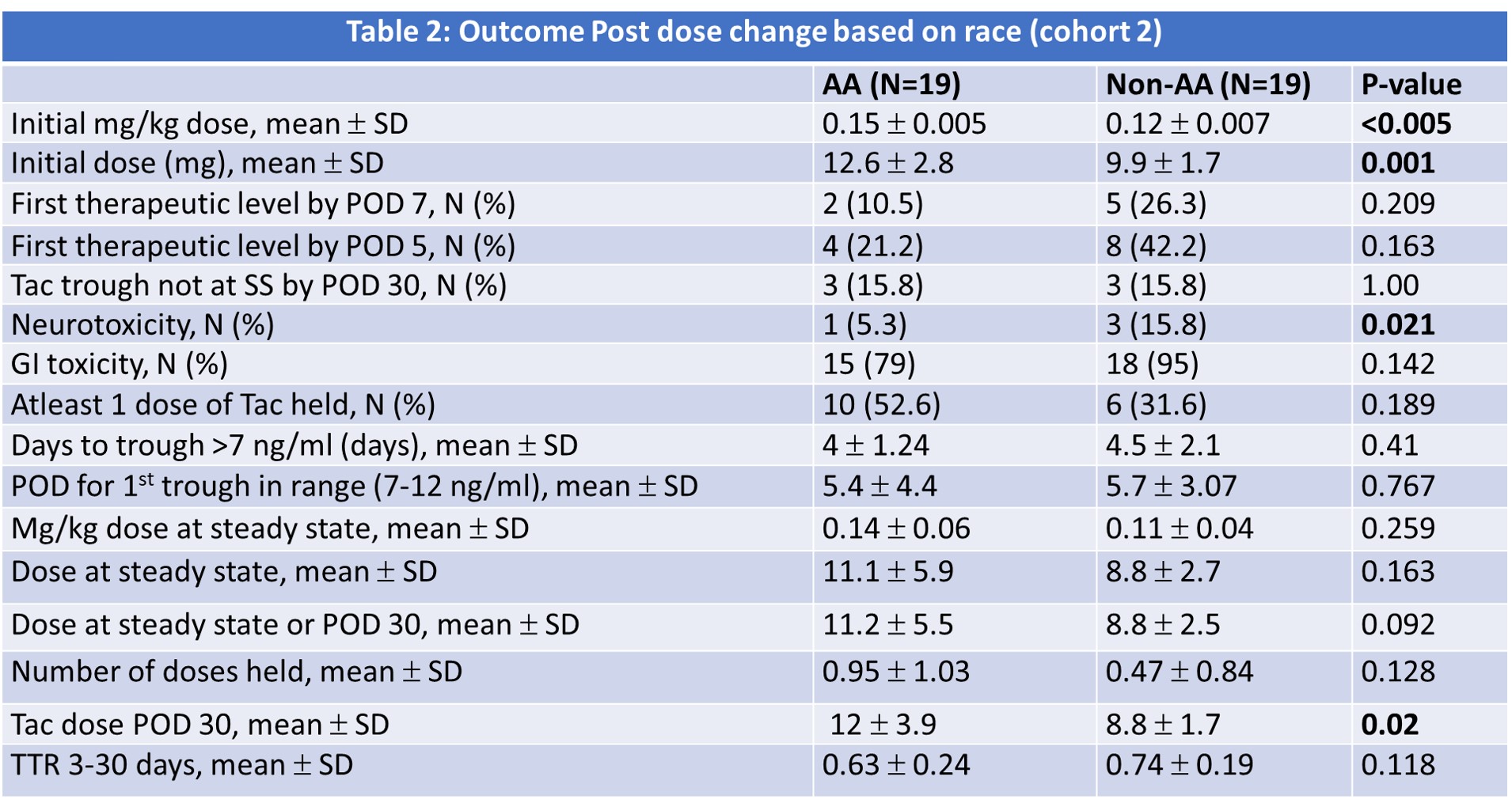
*Purpose: In May of 2020, our center was challenged with a nationwide shortage of tacrolimus IR, warranting implementation of a new de novo tacrolimus XR protocol. The purpose of this study is to assess two different dosing strategies of Tacrolimus XR in African American (AA) and non-AA de novo kidney transplant recipients.
*Methods: This retrospective study includes adult kidney transplant recipients between May 2020 and Sept 2020.The study population was divided into two groups across two different dosing strategies:non-AA and AA and then further stratified based on initial dosing.The initial dosing strategy was to give all patients between 0.12mg/kg and 0.17mg/kg, regardless of race; the second strategy was to give 0.12 mg/kg to non-AAs and 0.15mg/kg to AAs. The primary endpoint was days to a therapeutic tac trough (>7 ng/ml).Other endpoints included dose at steady state (SS), number of doses held, dose at POD 30,time in therapeutic range (TTR) in the first month and adverse effects.
*Results: A total of 122 patients were included. There was a statistically higher number of DDKT and DCD in the AA cohort. They also had a significantly higher EPTS score, HLA mismatches, longer time on dialysis, and were more likely to get induction with ATG. In initial analysis, there was a significant interaction between race and dosing strategy for the outcome of time to therapeutic level. During the initial dosing strategy, AA had a significantly longer time to achieving a therapeutic trough (6.2 vs. 4.4 days, P value 0.03). The non-AA group experienced a higher incidence of neurotoxicity compared to the AA group. After the dosing strategy was changed for AA recipients, the time to a therapeutic level decreased to 4 days with no significance between groups. The incidence of neurotoxicity continued to be higher in the non-AA group. The remining results are shown in table 1 and 2.
*Conclusions: The results demonstrate that AAs can achieve a similar time to therapeutic tac trough concentrations with tacrolimus XR, as compared to non-AAs, using a race stratified dosing strategy.Future analyses are underway to assess the impact of CYP 3A5 genotype on dose requirements and time to achieve therapeutic levels.
To cite this abstract in AMA style:
Patel N, Carcella T, Bartlett F, Rohan V, Taber D. Racial Differences in Tacrolimus Xr Dosing in De Novo Kidney Transplant Recipients [abstract]. Am J Transplant. 2021; 21 (suppl 3). https://atcmeetingabstracts.com/abstract/racial-differences-in-tacrolimus-xr-dosing-in-de-novo-kidney-transplant-recipients/. Accessed February 26, 2026.« Back to 2021 American Transplant Congress