Quantiferon-tb Gold Plus in Liver Transplant Candidates: Single-center Experience
J. Simkins1, M. A. Mendoza1, A. Chandorkar1, Y. Natori1, S. Anjan1, L. R. Arosemena1, R. Vianna2
1Medicine, University of Miami, Miami, FL, 2Surgery, University of Miami, Miami, FL
Meeting: 2021 American Transplant Congress
Abstract number: 769
Keywords: Infection, Liver transplantation, N/A, Radiologic assessment
Topic: Clinical Science » Infectious Disease » All Infections (Excluding Kidney & Viral Hepatitis)
Session Information
Session Name: All Infections (Excluding Kidney & Viral Hepatitis)
Session Type: Poster Abstract
Session Date & Time: None. Available on demand.
Location: Virtual
*Purpose: QuantiFERON-TB (QFT) is the preferred diagnostic test for latent tuberculosis infection (LTBI). The QFT-Gold Plus (QFT-Plus) data in OLT candidates is limited.
*Methods: Our hospital replaced QFT-Gold In-Tube (QFT-GIT) with QFT-Plus in 3/2019. We assessed QFT-Plus results performed prior to OLT among patients that were transplanted between 4/2019 and 8/2020 at a large transplant center. We obtained previous QFT-GIT results if available to assess for discordant results. Chest-x-rays (CXR) and chest CT scans performed within 1 year prior to OLT were obtained to assess for LTBI signs (nodules, pleural thickening, and scarring). The plan by the infectious diseases (ID) team for those OLT patients with positive or indeterminate QFT-Plus was evaluated.
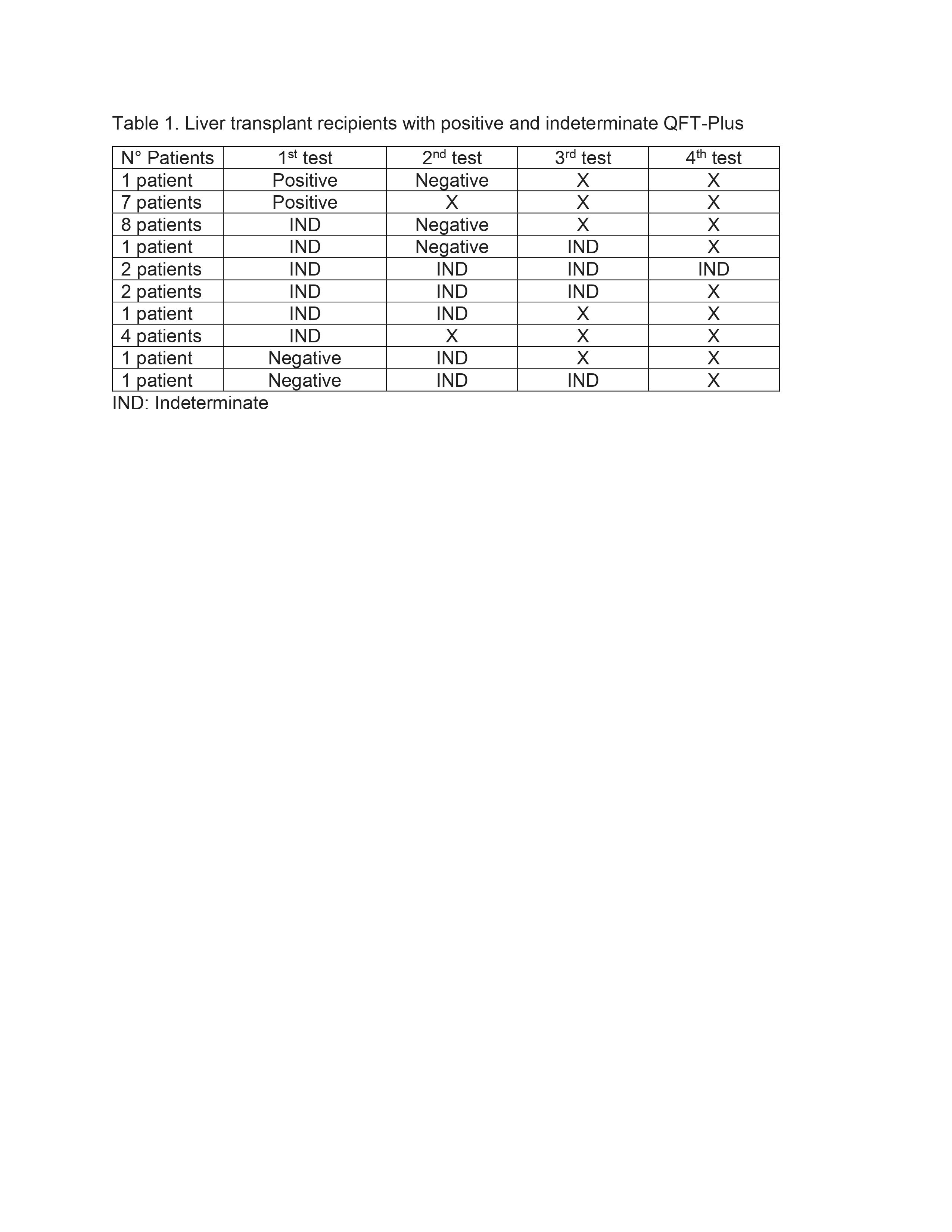
*Results: We assessed 170 OLT recipients [122(72%) Male, 94(55%) Hispanic]. Median age was 58 (range: 21-75) years. QFT-Plus was performed in 124(73%) patients [86(69%) were tested once, 28(23%) twice, 8(6%) thrice and 2(2%) four times]. Eight out of 124(6%) had positive, 20(16%) indeterminate and 96 (77%) negative QFT-Plus. One of the QFT-Plus-positive patients, tested negative 9 days after testing positive, and 9(45%) of the QFT-Plus-indeterminate patients converted to negative (Table 1). Previous QFT-GIT were performed in three QFT-Plus-positive patients (it was positive in 2 and negative in 1 ), in two QFT-Plus-indeterminate patients (it was negative in both), and in 16 QFT-Plus-negative patients (it was positive in 1 and negative in 15). There was no difference in the prevalence of LTBI-suggestive radiographic findings between patients with positive and negative QFT-Plus [CXR: 1/8(13%) vs. 8/96(8%), P=0.53 and CT scan: 4/5(80%) vs. 32/35(91%), P=0.43]. ID team recommended isoniazid for 9 months for 7(88%) and 9(45%) patients with positive and indeterminate QFT-Plus, respectively. Isoniazid was not recommended in 1 case of positive QFT-Plus (previously treated).
*Conclusions: QFT-Plus appears to be an acceptable test for LTBI diagnosis in OLT candidates. In our cohort, indeterminate QFT-Plus was common. QFT-Plus conversion from indeterminate to negative was frequent.
To cite this abstract in AMA style:
Simkins J, Mendoza MA, Chandorkar A, Natori Y, Anjan S, Arosemena LR, Vianna R. Quantiferon-tb Gold Plus in Liver Transplant Candidates: Single-center Experience [abstract]. Am J Transplant. 2021; 21 (suppl 3). https://atcmeetingabstracts.com/abstract/quantiferon-tb-gold-plus-in-liver-transplant-candidates-single-center-experience/. Accessed March 7, 2026.« Back to 2021 American Transplant Congress