Protocolized, Broad-Based Renal Genetic Testing in the Management of Patients at a Transplant Center
1Baystate Medical Center, Springfield, MA, 2Natera, Inc., Austin, TX, 3Renal Transplant Associates of New England, Springfield, MA
Meeting: 2022 American Transplant Congress
Abstract number: 506
Keywords: Gene polymorphism, Kidney
Topic: Clinical Science » Kidney » 49 - Recurrent Kidney Disease & Genetics
Session Information
Session Name: Recurrent Kidney Disease & Genetics
Session Type: Rapid Fire Oral Abstract
Date: Tuesday, June 7, 2022
Session Time: 5:30pm-7:00pm
 Presentation Time: 6:40pm-6:50pm
Presentation Time: 6:40pm-6:50pm
Location: Hynes Room 310
*Purpose: Studies show that ~10% of adult chronic kidney diseases (CKD) cases have genetic etiologies. In the nephrology setting, the use of a broad, unbiased genetic panel may have benefit over using smaller, targeted panels based on the clinical presentation. Here we describe the use of an NGS-based panel of 382 or 385 genes related to kidney disease (the RenasightTM test), in our transplant nephrology center.
*Methods: From November 2020-2021 our center incorporated genetic testing for select donors, patients with CKD, and kidney transplant (KT) recipients. Patients were offered genetic testing if they met one of the following criteria: (i) KT recipient, (ii) history of undiagnosed kidney disease, (iii) family history of kidney disease or (iv) African American ancestry. Prospective donors were offered testing if there was a family history of kidney disease and/or if they were of African American descent. The presence of 1 pathogenic (P)/likely pathogenic (LP) variant in an X-linked or autosomal dominant condition, 2 P/LP variants in an autosomal recessive condition, or 2 APOL1 G1 or G2 alleles were considered positive findings.
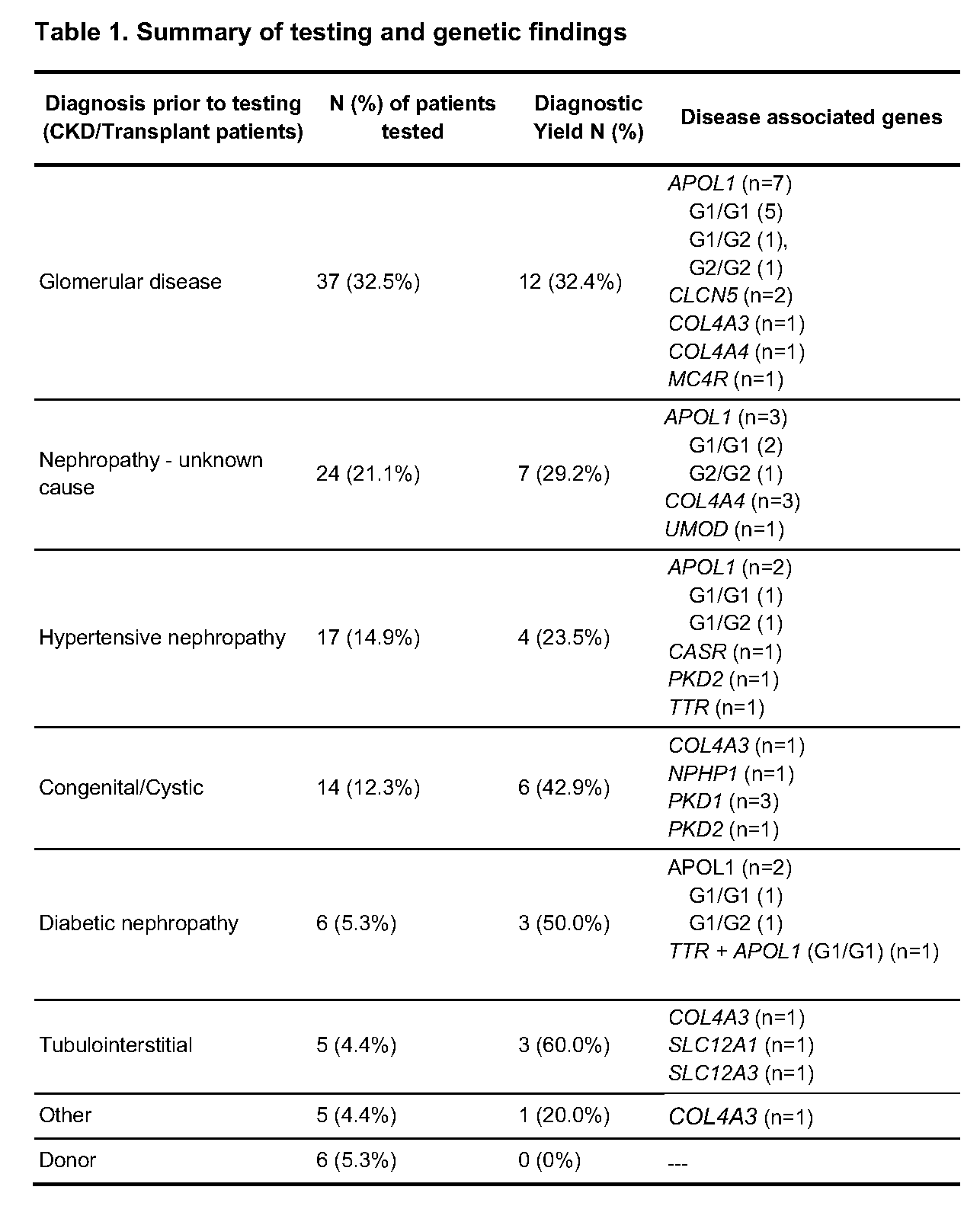
*Results: A total of 114 patients fit the testing criteria (KT recipients: n=33, CKD/waitlisted: n=75; donors: n=6), of which, 56% were male (n=62) with an average age of 49 years (range: 7-81). The clinical diagnoses of the tested CKD and KT patients were classified in 7 categories (Table 1); of which, the most common were glomerular disease (n=37, 32.46%), nephropathy of unknown cause (n=24, 21.05%) and hypertensive nephropathy (n=17, 14.91%). Overall, 31.6% (36/114) patients had positive findings in 13 genes; 2 patients had dual positive results (Table 1). None (0/6) of the prospective donors had positive findings. Notably, genetic findings lead to disease reclassification or immediate change in management in 29 of the 108 CKD/KT patients.
*Conclusions: Incorporating broad panel genetic testing into clinical practice at a transplant nephrology center yielded valuable genetic insights for patients with CKD, KT and potential donors. In multiple affected patients, the clinical presentation did not align with the genetic diagnosis, leading to reclassification of disease. Unanticipated negative results for some patients prompted additional workup. This experience demonstrates that implementing protocolized genetic testing with a broad gene panel can identify patients with CKD for which testing is appropriate.
To cite this abstract in AMA style:
Paramasivam V, Shehzad P, Greco B, Gaj K, Germain M. Protocolized, Broad-Based Renal Genetic Testing in the Management of Patients at a Transplant Center [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/protocolized-broad-based-renal-genetic-testing-in-the-management-of-patients-at-a-transplant-center/. Accessed February 27, 2026.« Back to 2022 American Transplant Congress