Preliminary Experience Utilizing Boceprevir with Pegylated Interferon and Ribavirin for Treatment of Recurrent Hepatitis C Genotype 1 after Liver Transplantation
Gastroenterology and Hepatology, Mayo Clinic Arizona, Phoenix
Meeting: 2013 American Transplant Congress
Abstract number: 365
Background Hepatitis C virus (HCV) recurrence after liver transplant is universal and causes significant reduction in both graft and patient survival. The use of protease inhibitors combined with pegylated interferon (PEG) and RBV significantly improve sustained virological response in non-LT patients. There is limited experience regarding their use post LT.
Aim: To describe our experience with Boceprevir-based triple therapy for recurrent HCV post LT
Methods: Single center study describing the outcome of HCV treatment post LT, utilizing a strict and standardized clinical protocol.
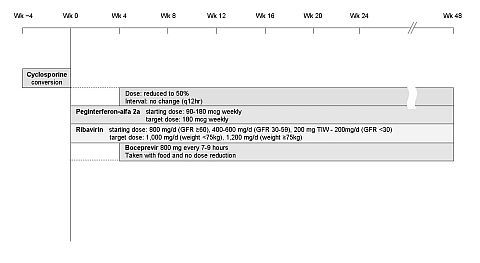
IS levels were closely monitored once Boceprevir was initiated. Demographic, clinical, biochemical and virological data was collected at baseline and during therapy. Ribavirin levels were monitored every 4 weeks
Results: Total of 16 patients with genotype 1 recurrent HCV were treated with Boceprevir bases regimen between 06/11-10/12. Treatment characteristics are shown in Table 1.
| Boceprevir based treatment (n=16) | |
| Age (years) | 59 |
| Gender: Male | 15 (94%) |
| HCV Genotype | |
| – 1a | 12(75%) |
| – 1b | 3(19%) |
| – 1 | 1 (6%) |
| IL28b Polymorphism | |
| – CC | 2 (13%) |
| – CT | 9 (56%) |
| – TT | 5 (21%) |
| Duration of Treatment (weeks) | 16 (4-48) |
| – Passed week 4 | 16(100%) |
| – Passed week 12 | 12(75%) |
| – Passed week 24 | 5(31%) |
| – Passed week 48 | 1(6%) |
Four patients (25%) achieved complete early virological response; five patients (31%) are negative at week 24. Six (37.5%) met the futility rules and showed inadequate virological response. One patient (6%) stopped treatment at week 4 due to severe ribavirin related rash. All patients required growth factor support on treatment.
Conclusions: Boceprevir based triple therapy post LT but requires close clinical monitoring. Interactions with IS were managed successfully with close monitoring. Though efficacy of treatment is better than standard therapy, response rates are still suboptimal. Patients should be closely monitored for cytopenias. The incremental side effects of this interferon-based regimen continues to limit access and viral clearance.
To cite this abstract in AMA style:
Aqel B, Carey E, Douglas D, Byrne T, Rakela J, Susan W, Vargas H. Preliminary Experience Utilizing Boceprevir with Pegylated Interferon and Ribavirin for Treatment of Recurrent Hepatitis C Genotype 1 after Liver Transplantation [abstract]. Am J Transplant. 2013; 13 (suppl 5). https://atcmeetingabstracts.com/abstract/preliminary-experience-utilizing-boceprevir-with-pegylated-interferon-and-ribavirin-for-treatment-of-recurrent-hepatitis-c-genotype-1-after-liver-transplantation/. Accessed July 18, 2025.« Back to 2013 American Transplant Congress
