Predicting Transplant Rejection by a Composite Urinary Injury Score
Department of Surgery, UCSF, San Francisco, CA
Meeting: 2019 American Transplant Congress
Abstract number: 466
Keywords: Kidney transplantation, Monitoring, Prognosis, Rejection
Session Information
Session Name: Concurrent Session: Biomarkers, Immune Monitoring and Outcomes IV
Session Type: Concurrent Session
Date: Tuesday, June 4, 2019
Session Time: 2:30pm-4:00pm
 Presentation Time: 2:30pm-2:42pm
Presentation Time: 2:30pm-2:42pm
Location: Room 306
*Purpose: While sequencing or SNP-based methods of quantifying donor-derived cell-free DNA (dd-cfDNA) from plasma have been used to detect allograft rejection, they remain expensive and inconvenient for patients and physicians. We assessed the performance of a novel urinary assay to provide a quantitative composite risk score of DNA and protein markers without the need for sequencing or SNPs to detect kidney transplant rejection (KT) rejection.
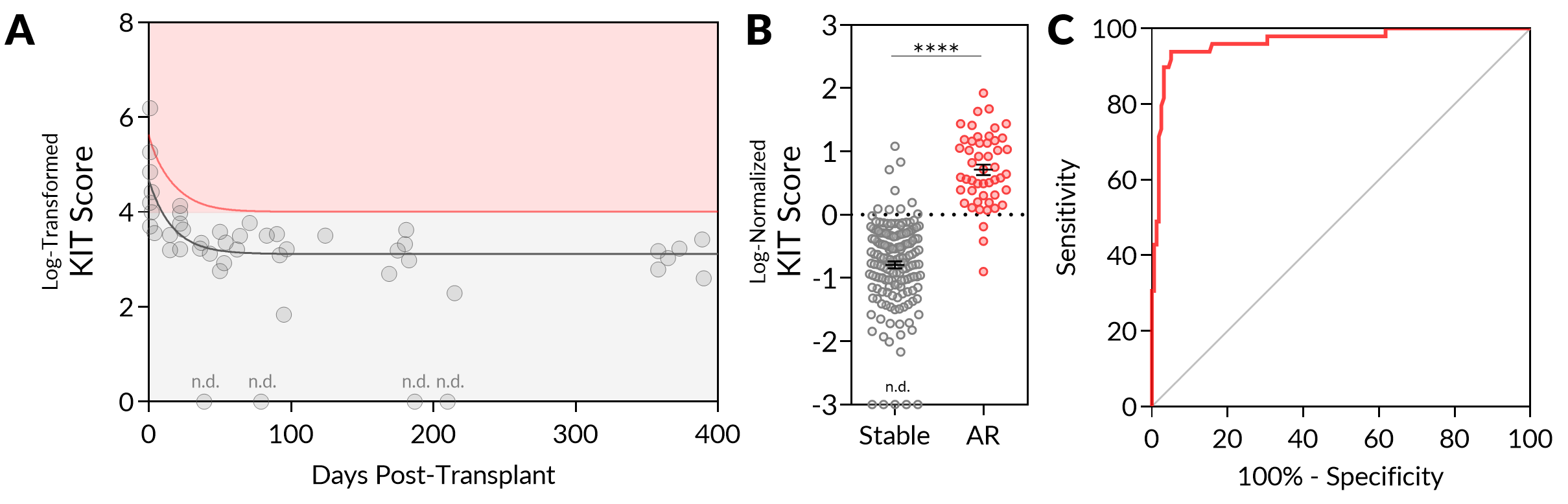
*Methods: 206 unique urine samples from KT patients were collected and categorized as stable (n = 157) or acute rejection (AR, n = 49). Samples were processed for quantification of urinary donor-derived cfDNA (genomic equivalents (GE)/mL) and 5 additional protein markers using a custom microcell-based assay, to develop a Kidney Injury Test (KIT) score. The KIT score from longitudinally collected samples (n = 47) from 8 KT patients who were stable and had no evidence of subclinical rejection was correlated with days post-transplant to generate a one-sided, one-phase decay 95% prediction curve which was used to generate a normalized KIT score for all samples.
*Results: The urinary KIT score is significantly increased immediately post-transplant and decreases to a steady baseline by 3 months post-transplant (Figure 1A). The median level of 95% prediction interval-normalized KIT score was significantly higher in AR as compared with stable samples (0.64 vs. -0.71, P < 0.0001) (Figure 1B). The urinary KIT score showed high performance in discriminating the stable and AR samples, with an AUC of 0.9649 (P < 0.0001). At a threshold set at a normalized value of 0, the sensitivity and specificity of the assay was 93.88% and 94.90% respectively (Figure 1C), suggesting that the assay could be used to non-invasively screen patients at risk of rejection to avoid unnecessary biopsies in the clinical setting.
*Conclusions: This novel urinary KIT score enables rapid and accurate discrimination of AR from stable patients with results returnable within the same day of urine sample receipt and without the costs associated with sequencing. As collection of urine requires no training and can be performed as often as needed, this assay can provide inexpensive, accurate, and longitudinal assessment of AR in KT patients in a format amenable to use in transplant clinics.
To cite this abstract in AMA style:
Yang JY, Sarwal R, Sigdel TK, Sarwal MM. Predicting Transplant Rejection by a Composite Urinary Injury Score [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/predicting-transplant-rejection-by-a-composite-urinary-injury-score/. Accessed March 4, 2026.« Back to 2019 American Transplant Congress