Predicted Performance of Bayesian-Based Precision Dosing Software for Tacrolimus
1University of California San Diego Health, San Diego, CA, 2PrecisePK, San Diego, CA, 3UCSD Skaggs School of Pharmacy and Pharmaceutical Sciences, San Diego, CA
Meeting: 2022 American Transplant Congress
Abstract number: 531
Keywords: Calcineurin, Kidney transplantation, Pharmacokinetics
Topic: Clinical Science » Pharmacy » 29 - Non-Organ Specific: Pharmacokinetics / Pharmacogenomics / Drug interactions
Session Information
Session Name: Non-Organ Specific: Pharmacokinetics / Pharmacogenomics / Drug interactions
Session Type: Rapid Fire Oral Abstract
Date: Tuesday, June 7, 2022
Session Time: 5:30pm-7:00pm
 Presentation Time: 6:20pm-6:30pm
Presentation Time: 6:20pm-6:30pm
Location: Hynes Room 311
*Purpose: Bayesian-based precision dosing software (BPDS) has been utilized for therapeutic drug monitoring to optimize medication use. However, BPDS for tacrolimus (TAC) is not routinely utilized in clinical settings. The objective of this study was to evaluate the predictive performance of BPDS for TAC in early post-kidney transplant recipients (KTR).
*Methods: A single-center, retrospective cohort study was performed among KTR between 01/2018 – 12/2019 with TAC dosing and trough concentrations (C0) during index hospitalization. The predictive performance of two models were evaluated within the BPDS with the number TAC C0 varied per patient. Model 1 covariates included: prednisolone dose, CYP3A5 expression, body size and post-operative day (POD). Model 2 covariates included: CYP3A5 and hematocrit. Predictive performance was determined by assessing both bias (calculated as the mean difference between observed and predicted TAC C0 and mean absolute prediction error (MAPE)) and precision (measured as the standard deviation (SD) of the difference).
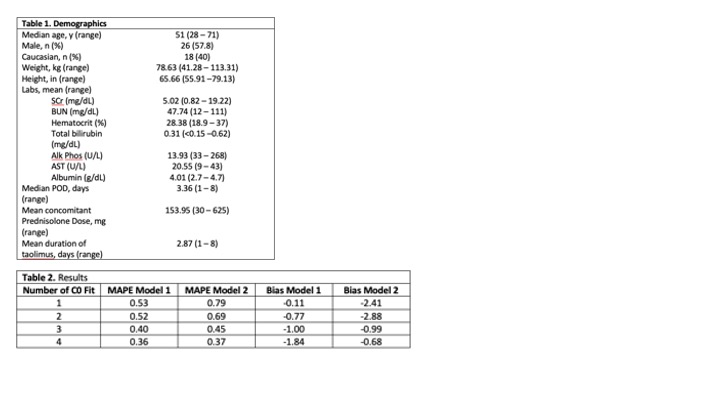
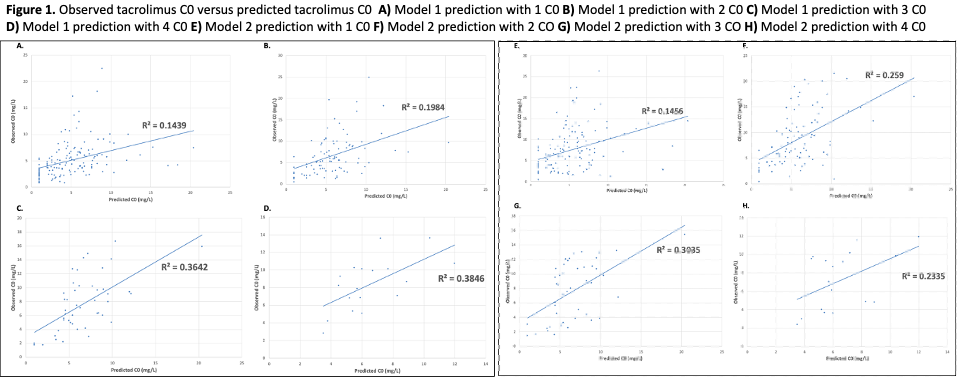
*Results: There were 45 patients with 145 TAC C0 included. Baseline demographics are shown in Table 1. Compared to Model 2 (Figure), Model 1 had a higher predictive performance when fitting a lower number of TAC C0. Both models approximated one another at C0 >2. MAPE improved for both models as additional C0 were included as seen in Table 2. Bias for both models using mean difference was relatively low (-0.11 to 2.88 mg/L) however both models showed low precision in terms of SD (2.36 to 4.50 mg/L).
*Conclusions: BPDS may help guide TAC dosing in early post-KTP. Model 1 appeared to have better predicted C0 when compared with model 2. Precision improved for both models as more C0 were incorporated. The use of BPDS could lead to less frequent TAC C0 monitoring.
To cite this abstract in AMA style:
Seghezzo A, Kerr J, Gupta A, Ta A, Powell L, Feist A. Predicted Performance of Bayesian-Based Precision Dosing Software for Tacrolimus [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/predicted-performance-of-bayesian-based-precision-dosing-software-for-tacrolimus/. Accessed February 18, 2026.« Back to 2022 American Transplant Congress