Pre-Emptive Rituximab to Prevent Post Transplant Lymphoproliferative Disorder (PTLD) in Pediatric Kidney Transplant Recipients with EBV DNAemia: A Case Series from the Pediatric Nephrology Research Consortium (PNRC)
I. Ashoor1, S. Ranabothu2, S. Al-Akash3, A. Moudgil4, Y. Shi4, S. Kizilbash5, D. Puliyanda6, V. Dharnidharka7
1LSU Health Sciences Center New Orleans, New Orleans, LA, 2Arkansas Children's Hospital, Little Rock, AR, 3Driscoll Children's Hospital, Corpus Christie, TX, 4Children's National Medical Center, Washington, DC, 5University of Minnesota Medical Center, Minneapolis, MN, 6Cedars-Sinai Medical Center, Los Angeles, CA, 7Washington University in St. Louis, St Louis, MO
Meeting: 2022 American Transplant Congress
Abstract number: 105
Keywords: Epstein-Barr virus (EBV), Kidney transplantation, Pediatric, Post-transplant lymphoproliferative disorder (PTLD)
Topic: Clinical Science » Kidney » 43 - Kidney: Pediatrics
Session Information
Session Time: 5:30pm-7:00pm
 Presentation Time: 5:40pm-5:50pm
Presentation Time: 5:40pm-5:50pm
Location: Hynes Ballroom C
*Purpose: The use of Rituximab (RTX) to prevent PTLD in patients with EBV DNAemia is an established strategy in hematopoietic stem cell transplants, but with scant data in solid organ transplants. Here we describe the largest cohort of pediatric kidney transplant recipients (KTR) with EBV DNAemia treated with RTX to prevent PTLD.
*Methods: KTR with EBV DNAemia treated with RTX to prevent PTLD between 7/1999 and 7/2019 at 5 PNRC centers were included and those with confirmed PTLD at the onset of RTX were excluded.
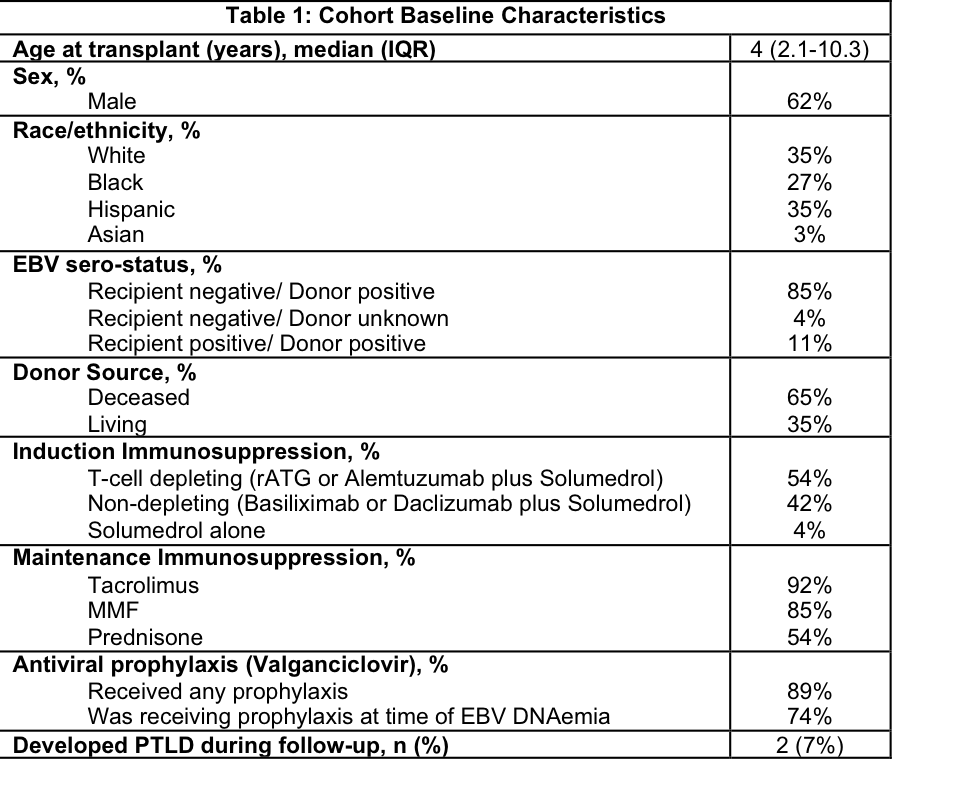
*Results: Of 26 KTRs (Table); 17 (65%) were <6 years at transplant. EBV DNA load monitoring by qPCR was performed at 1-3 month intervals. EBV DNAemia onset occurred at a median 73 days post-transplant (IQR 52-307), followed by DNAemia peak at a median of 268 days (IQR 112-536). RTX was administered at a median of 9 days post peak (IQR 0-118). RTX regimens varied; median dose 375 mg/m2 (IQR 375-439) weekly for 1-4 doses per course. Adverse reactions included hypogammaglobulinemia (n=3), mild allergic reaction, pneumonia, and anaphylaxis (each n=1).
Following RTX, EBV DNA load decreased to < 10% of baseline at 120 days in 20/26 patients, however, only 30% achieved complete resolution at last follow-up. Repeat courses of RTX were given to 8 patients with recurrent DNAemia.
At last follow-up (median 2094 days post-transplant [IQR 1538-3463]), all KTR had functioning grafts, 1 death occurred in a child with PTLD following remission due to reasons unrelated to PTLD. Two (7%) developed PTLD at an average of 1314 days post RTX. Both had received one course of 4 weekly RTX infusions and had <10% baseline EBV PCR load 1 year post RTX. One case involved sigmoid colon (Burkitts), and required immunosuppression reduction, RTX, and chemotherapy to induce remission. The other involved tonsillar tissue and achieved remission with tonsillectomy.
*Conclusions: In the largest pediatric KTR cohort with EBV DNAemia given RTX to prevent PTLD, RTX achieved a short-term reduction in DNA load, however, recurrent DNAemia is common. A randomized trial design is needed to determine the safety and efficacy of pre-emptive RTX to prevent PTLD in this population.
To cite this abstract in AMA style:
Ashoor I, Ranabothu S, Al-Akash S, Moudgil A, Shi Y, Kizilbash S, Puliyanda D, Dharnidharka V. Pre-Emptive Rituximab to Prevent Post Transplant Lymphoproliferative Disorder (PTLD) in Pediatric Kidney Transplant Recipients with EBV DNAemia: A Case Series from the Pediatric Nephrology Research Consortium (PNRC) [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/pre-emptive-rituximab-to-prevent-post-transplant-lymphoproliferative-disorder-ptld-in-pediatric-kidney-transplant-recipients-with-ebv-dnaemia-a-case-series-from-the-pediatric-nephrology-research-co/. Accessed March 9, 2026.« Back to 2022 American Transplant Congress