Post-Transplant Outcomes Comparing A2 Incompatible to Compatible Deceased Donor Liver Transplant Recipients
Department of Surgery, Johns Hopkins University School of Medicine, Baltimore, MD
Meeting: 2021 American Transplant Congress
Abstract number: 1139
Keywords: Histocompatibility
Topic: Clinical Science » Liver » Liver: MELD, Allocation and Donor Issues (DCD/ECD)
Session Information
Session Name: Liver: MELD, Allocation and Donor Issues (DCD/ECD)
Session Type: Poster Abstract
Session Date & Time: None. Available on demand.
Location: Virtual
*Purpose: Transplantation of A2 livers into type O and B recipients (A2i DDLT) is a growing modality to increase access to DDLT for O and B candidates. Most studies on the safety of A2i DDLT in conjunction with their induction agents have been single-center. We sought to explore A2i DDLT outcomes on a national scale.
*Methods: Using SRTR data 1/2005-9/2020, we compared acute rejection and graft and patient survival in 763 A2i recipients vs 48,445 ABO-compatible (A2c) recipients using inverse probability of treatment weights to adjust for recipient confounders (demographics, BMI, cause of ESLD, MELD, calendar year). We compared outcomes stratified by induction agent (steroid only, thymoglobulin, basiliximab, others or multiple) among A2i.
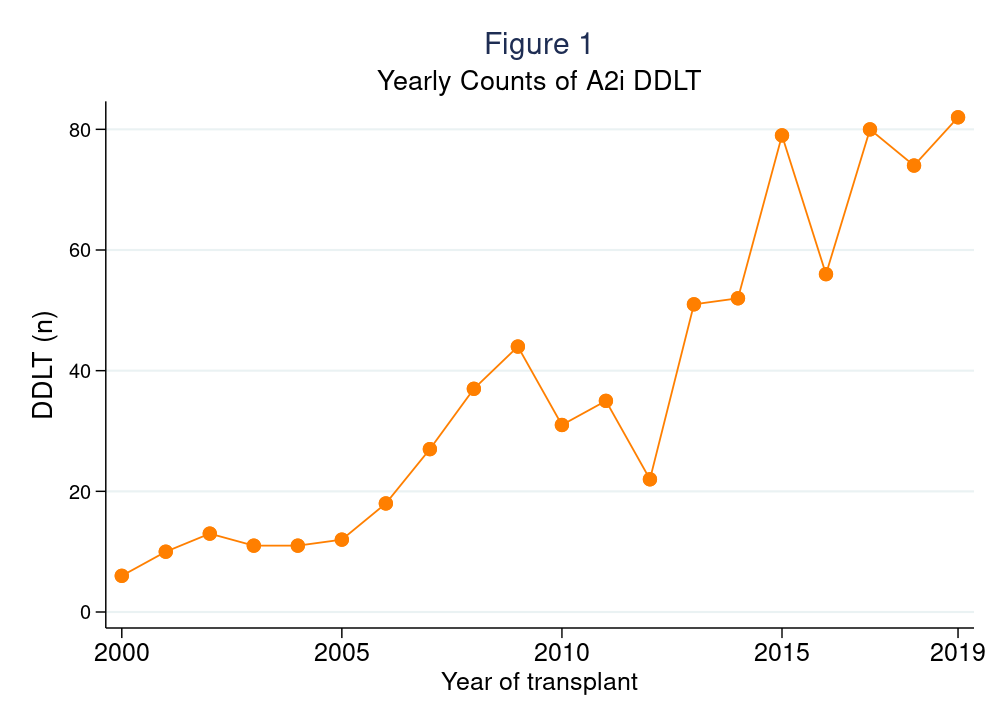
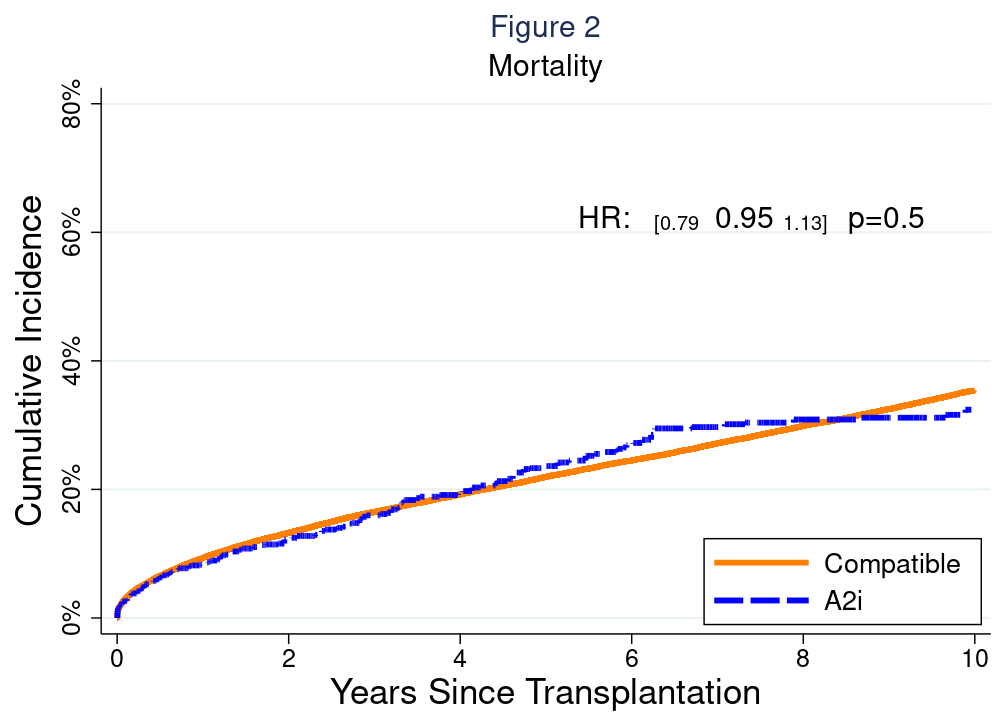
*Results: The number of A2i DDLT per year increased from 6 in 2000 to 82 in 2019 (Figure 1). A2i recipients spent less time on waitlist (A2i median (IQR) 60 (8-286) days vs A2c 89 (16-288) days, p<0.001) and had higher MELD at allocation (26 (15-34) vs. 20 (13-29), p<0.001). A2i recipients had longer length of stay after DDLT (12 (7-18)d vs 9 (7-16)d, p<0.001). Risk of acute rejection prior to discharge was higher among A2i (9.1% vs. 5.1%, weighted odds ratio (wOR)=1.461.962.64, p<0.001), but was otherwise comparable between the two groups at 6-months (9.4% vs. 8.7%, wOR=0.881.191.61, p=0.3), 1-year (12.3% vs. 11.6%, wOR=0.871.141.49, p=0.3), 2-year (14.7% vs. 13.6%, wOR=0.911.171.50, p=0.2) follow-up. Post-DDLT patient survival (wHR=0.790.951.13, p=0.5) and graft survival (wHR=0.840.991.17, p=0.9) were also comparable between groups (Figure 2). 16.1% and 11.7% of A2i received basiliximab and thymoglobulin as induction, respectively. There was no evidence of improved acute rejection or patient/graft survival for patients who received non-steroid induction agents.
*Conclusions: Utilization of A2i DDLT has increased substantially over time. Transplant candidates who accepted A2i DDLT had shorter time to transplant, and generally comparable outcomes with A2c recipients. Our data support the continued practice of A2i DDLT to increase access to DDLT for type O and B candidates.
To cite this abstract in AMA style:
Chiang T, Eagleson MA, Bae S, Garonzik-Wang J, Segev D, Massie AB. Post-Transplant Outcomes Comparing A2 Incompatible to Compatible Deceased Donor Liver Transplant Recipients [abstract]. Am J Transplant. 2021; 21 (suppl 3). https://atcmeetingabstracts.com/abstract/post-transplant-outcomes-comparing-a2-incompatible-to-compatible-deceased-donor-liver-transplant-recipients/. Accessed February 17, 2026.« Back to 2021 American Transplant Congress