Population Pharmacokinetics of Voclosporin: Body Surface Area Optimized Dosing
Isotechnika Pharma Inc, Edmonton, AB, Canada
Department of Medicine, University of Cincinnati, Cincinnati, OH
Meeting: 2013 American Transplant Congress
Abstract number: B1026
Objective:
To determine the population pharmacokinetics (popPK) of voclosporin (VCS), a new calcineurin inhibitor (CNi), and explore potential covariate relationships to individualize drug dosing.
Background:
Inter and intra-individual variability of highly lipophilic drugs is especially influenced by body composition. Obese patients and/or patients with hyperlipidemia may demonstrate elevations in drug distribution which may also be influenced by inter-compartmental transfer and clearance. VCS is a highly lipophilic drug which demonstrates multi-compartment pharmacokinetics with slow penetration into deep fatty tissue.
Methods:
Bayesian popPK was performed using VCS whole blood concentrations derived from 100 de novo renal allograft patients, 133 healthy volunteers, 25 patients with mild to severe renal failure, and 12 patients with mild to moderate hepatic failure (WinBugs v1.4).
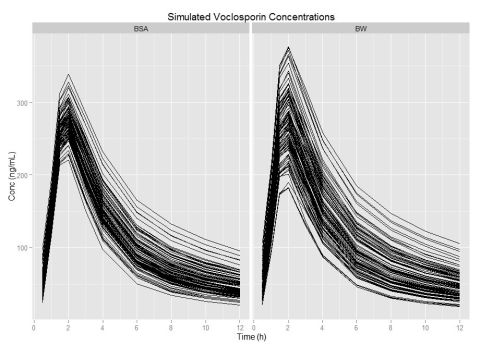
Results:
VCS popPK was well described by a 2-compartment model with first order absorptive input and time lag. Analysis indicates body weight, ideal body weight, body mass index, or allometric transforms of body weight were not statistically credible with little effect on drug clearance (CL/F) or distribution (V) suggesting weight based dosing does not minimize post-dose concentration variability. Interestingly, elevated triglycerides and dosing beyond the 28th day demonstrated a decrease in CL/F by factors of 0.95 and 0.84, respectively. Importantly, increasing body surface area (BSA) demonstrated a 2-fold increase in second compartment clearance (Q) and a 1.5 fold increase in first compartment (V1/F) volume that could be used to individualize drug therapy. As VCS is highly lipophilic, BSA may compensate for excess body fat composition.
Conclusions:
Model based simulations suggested that initial dose of 0.8 mg/kg or 34 mg/m2 achieved mean concentrations of 47.7 ng/mL and 47.6 ng/mL with percent coefficients of variation of 55.5% vs. 30.3%, suggesting that BSA-triglyceride-period-based dosing could decrease post-dose variability allowing more patients to achieve concentrations in the anticipated therapeutic range of 35 to 60 ng/mL early in the de novo transplant period.
Mayo, P.: Employee, Isotechnika. Ling, S.: Employee, Isotechnika.
To cite this abstract in AMA style:
Mayo P, Ling S, Alloway R. Population Pharmacokinetics of Voclosporin: Body Surface Area Optimized Dosing [abstract]. Am J Transplant. 2013; 13 (suppl 5). https://atcmeetingabstracts.com/abstract/population-pharmacokinetics-of-voclosporin-body-surface-area-optimized-dosing/. Accessed July 14, 2025.« Back to 2013 American Transplant Congress
