Pneumocystis Prophylaxis in Solid Organ Transplant Recipients at Two Large Transplant Centers from 2015-2016: Why Not Trimethoprim-Sulfamethoxazole?
1Infectious Diseases, Cleveland Clinic Floundation, Cleveland, OH, 2Infectious Diseases, University of Pennsylvania, Philadelphia, PA, 3Infectious Diseases, Cleveland Clinic Florida, Weston, FL
Meeting: 2019 American Transplant Congress
Abstract number: A344
Keywords: Adverse effects, Infection, Pneumonia, Prophylaxis
Session Information
Session Name: Poster Session A: Transplant Infectious Diseases
Session Type: Poster Session
Date: Saturday, June 1, 2019
Session Time: 5:30pm-7:30pm
 Presentation Time: 5:30pm-7:30pm
Presentation Time: 5:30pm-7:30pm
Location: Hall C & D
*Purpose: Trimethoprim-sulfamethoxazole (T/S) is the drug of choice for Pneumocystis jirovecii pneumonia (PJP) prophylaxis (ppx) after solid organ transplant (SOT). Perceived side effects may lead to the use of second line agents. We sought to describe the use of these agents across a variety of SOT programs.
*Methods: Pharmacy data was reviewed for all SOT recipients (SOTRs) at two large transplant centers from 1/2015-12/2016. Those prescribed an alternative prophylactic agent (APA) (pentamidine, atovaquone, or dapsone) within one year of SOT were included. Patient records were reviewed for descriptive characteristics, allergies, and laboratory values at the start of an APA. Positive diagnostic tests for T/S-preventable opportunistic infections (OIs) and APA -related side effects were collected.
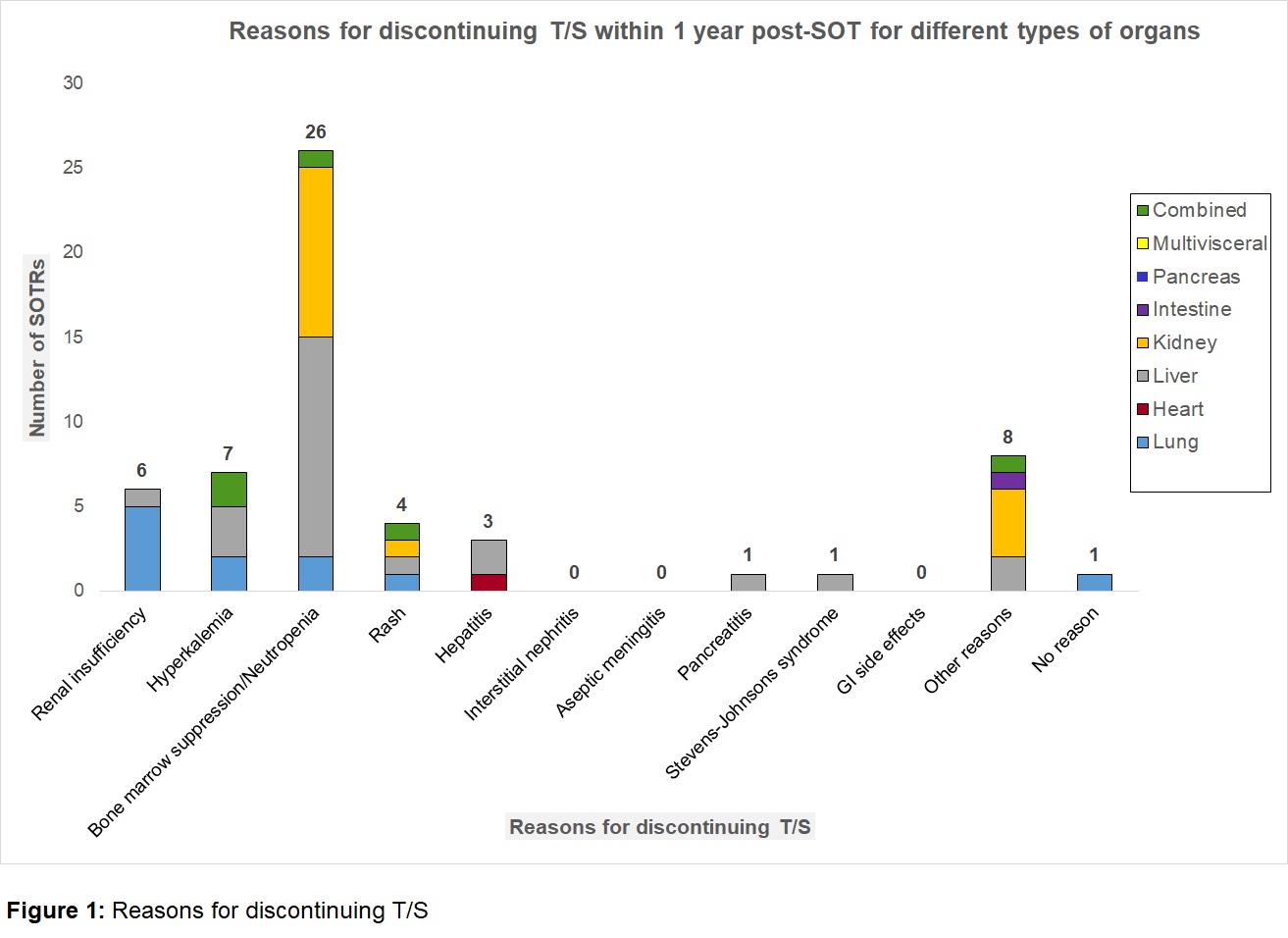
*Results: An APA was initiated in 105/1173 (8.9%) SOTRs. Of these, 51 (48.6%) were due to reported “sulfa moiety” allergy prior to SOT, mostly rash/hives (58.8%). 54 (51.4%) had T/S discontinued post-SOT, 94% within 6 months. Bone marrow suppression was the most common reason for stopping (48%), including 14 (53.8%) with an absolute neutrophil count >1000 cells/mm3. Nearly all of these 24/26 (92%) were also on mycophenolate mofetil, valganciclovir, or both agents. Other reasons for stopping initial T/S ppx are listed in Figure 1. Of note, 0 kidney transplant recipients had T/S discontinued for hyperkalemia or renal insufficiency. Nineteen SOTRs (35%) were later successfully re-challenged with T/S. On close review, it appeared that 62 (59%) were inappropriately changed to an APA (unlikely T/S allergy or attributable side effect). T/S-preventable OIs occurred in 7 SOTRs (5 Nocardia; 2 PJP), 3/7 occurred while on APA and 3/7 occurred on T/S. 1/7 was not on any PJP ppx at the time of infection. APA-related side effects (4 methemoglobinemia, 4 cytopenias, 1 rash) occurred in 10/105 (9.5%).
*Conclusions: PJP prophylaxis after SOT is frequently inappropriately changed from T/S, the drug of choice, to APAs. Such decisions should be more highly scrutinized to avoid T/S-preventable OIs and APA side effects. Guidance from experts in Hematology, Nephrology, and Allergy regarding rational thresholds for stopping T/S would improve use and patient outcomes.
To cite this abstract in AMA style:
Lum JM, Athans V, Echenique I, Koval CE. Pneumocystis Prophylaxis in Solid Organ Transplant Recipients at Two Large Transplant Centers from 2015-2016: Why Not Trimethoprim-Sulfamethoxazole? [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/pneumocystis-prophylaxis-in-solid-organ-transplant-recipients-at-two-large-transplant-centers-from-2015-2016-why-not-trimethoprim-sulfamethoxazole/. Accessed February 28, 2026.« Back to 2019 American Transplant Congress