Physician Survey on Using APOL1 Genetic Testing in Live Kidney Donor Selection
1Medicine, University of Michigan, Ann Arbor, MI, 2Surgery, Northwestern University, Chicago, IL, 3Medicine, Wake Forest, Winston-Salem, NC, 4Medicine, University of Iowa, Iowa City, IA, 5N/A, Miami, FL, 6Surgery, University of Alabama, Birmingham, AL
Meeting: 2020 American Transplant Congress
Abstract number: 241
Keywords: African-American, Genomics, Living donor, Screening
Session Information
Session Name: Kidney Living Donor: Selection
Session Type: Oral Abstract Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:45pm
 Presentation Time: 4:03pm-4:15pm
Presentation Time: 4:03pm-4:15pm
Location: Virtual
*Purpose: Live kidney donors of recent African ancestry are at an increased risk of developing end stage kidney disease than matched healthy non-donors. A recent small study suggests that homozygosity or compound heterozygosity for Apolipoprotein L1 (APOL1) renal risk variants G1 and G2 may explain this increased risk. In the absence of large studies verifying such an association, use of APOL1 genetic testing in selection of live kidney donor remains debatable. The American Society of Transplantation’s (AST) Living Donor Community of Practice (LDCoP) commissioned a work group to develop updated guidelines for APOL1 risk variant testing.
*Methods: We surveyed 322 physician members of the AST’s LDCoP about their practices around APOL1 genetic testing including: timing of testing, and use of test results in accepting potential live kidney donors. We used descriptive statistics to analyze the data. The study was approved by the IRB at the University of Michigan.
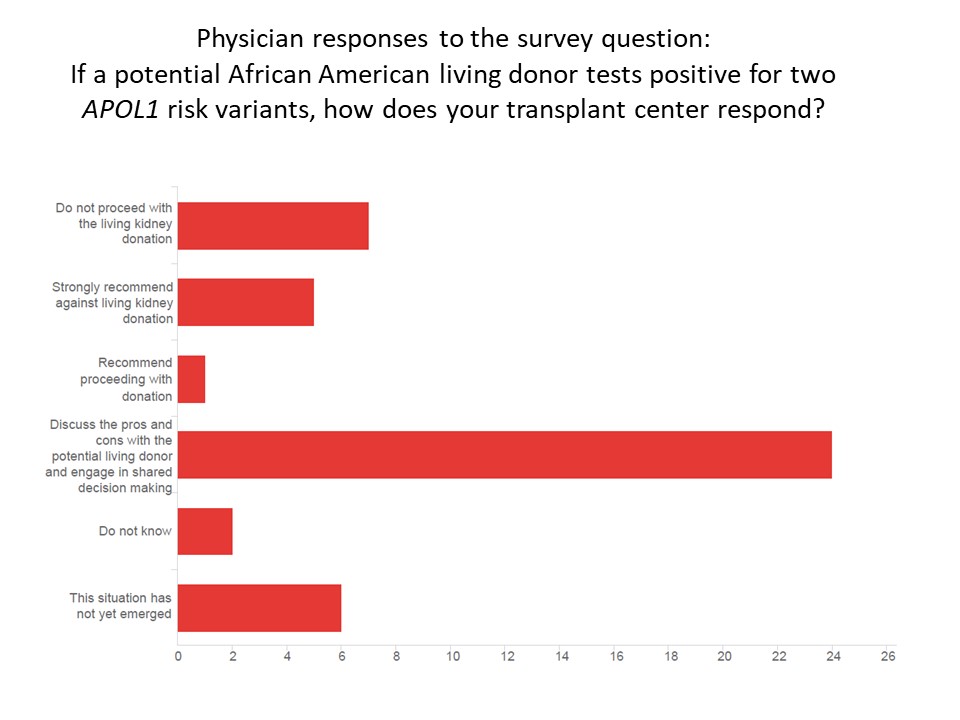
*Results: Eighty-two members (25%) completed the survey. Of these, most (57%) inform potential living donors about APOL1 testing, and the remaining 25 (43%) do not. Among the 47 physicians who discuss genetic testing, a third (32%) offer testing to all donors, half (53%) offer testing to selected donors, and few (15%) do not test anyone. Testing is offered most commonly when the donor is medically and socially cleared, but prior to final approval for donation (50%), others offer testing early in screening the donor for eligibility (25%), and others offer it later after preliminary medical testing or at evaluation (25%). In case of positive test results, most engage in shared decision making (51%), while few (16%) do not proceed with donation [see figure below].
*Conclusions: Most physicians inform potential live kidney donors about the availability of APOL1 risk variant testing. There is wide variation in clinical use and timing of APOL1 testing during the donor selection process. Our findings underscore the need for an ethical and clinically responsible approach to using APOL1 testing in clinical practice until definitive guidance from APOLLO and its ancillary studies become available in the future.
To cite this abstract in AMA style:
Doshi M, Gordon E, Freedman B, Thomas C, Glover C, Locke J. Physician Survey on Using APOL1 Genetic Testing in Live Kidney Donor Selection [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/physician-survey-on-using-apol1-genetic-testing-in-live-kidney-donor-selection/. Accessed March 1, 2026.« Back to 2020 American Transplant Congress