Pharmacy Led Initiatives for Treatment of Transplant Recipients of Hepatitis C Viremic Donors in a High Volume Transplant Center
H. Berry, J. Byrns, L. Crona, T. Capes, J. M. Steinbrink, E. K. Maziarz, C. R. Wolfe
Duke University Medical Center, Durham, NC
Meeting: 2020 American Transplant Congress
Abstract number: 283
Keywords: Hepatitis C, Infection, Pharmacoeconomics, Viral therapy
Session Information
Session Name: All Organs: Viral Hepatitis
Session Type: Oral Abstract Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:45pm
 Presentation Time: 4:15pm-4:27pm
Presentation Time: 4:15pm-4:27pm
Location: Virtual
*Purpose: We describe a multidisciplinary workflow for initiation of hepatitis C virus (HCV) direct-acting antiviral (DAA) therapies post-transplant in the outpatient setting of a high volume transplant program and we highlight the degree of pharmacy involvement for timely initiation of DAAs in a real-world setting.
*Methods: Retrospective chart review was performed, with data abstraction for all solid organ transplant (SOT) recipients of HCV-nucleic acid test (NAT)-positive organs. Date of transplant, treatments prescribed, treatment approval and start dates, and payer information was obtained.
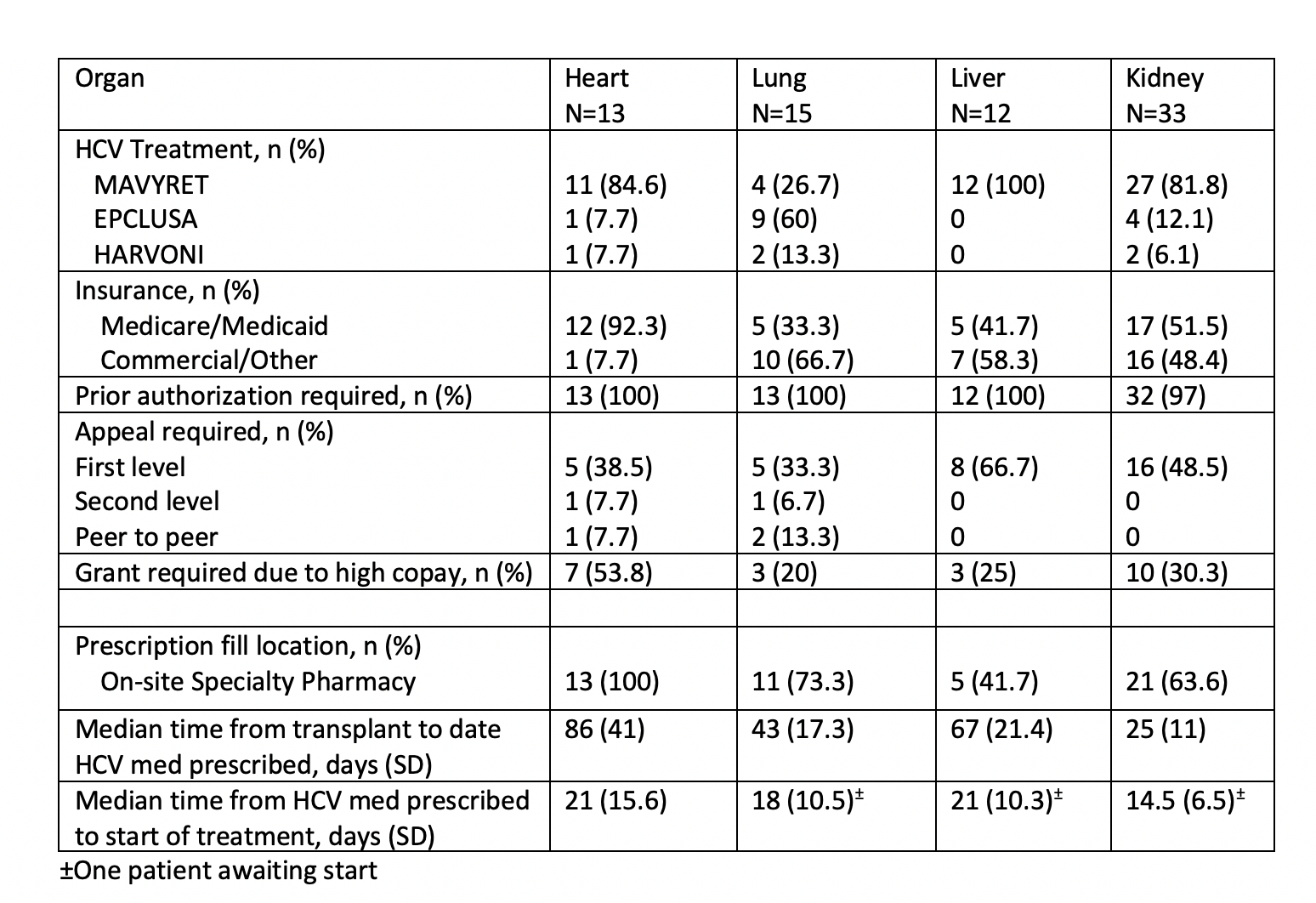
*Results: A standardized institutional pathway was followed for all organ systems, under IRB approval. A multidisciplinary outpatient team including a pharmacist, transplant physician, and transplant infectious diseases physician determine choice of therapy. A prescription is sent to the on-site specialty pharmacy and is processed by a dedicated pharmacy technician. The clinic pharmacist performs comprehensive drug interaction review, medication counseling, and notifies the team when treatment is initiated. Table 1 summarizes treatment details for 73 patients. Prior authorization was required for all but one prescription and 34 prescriptions (45%) needed further appeal. 35% of patients required financial assistance to initiate treatment given copay demands. 1 patient was denied approval for medication despite peer-to-peer review, and our institution supported their complete medication cost, per our protocol. Collating all pharmacy effort, a median estimated 2.8 hours of work per patient was required for a total of 204.5 hours.
*Conclusions: Complex coordination of care and multidisciplinary effort is required for initiating DAA treatment in SOT recipients of HCV NAT-positive organs, even at a high-volume transplant center. Wide variability exists in time from transplant to post-discharge medication initiation, as well as cost. Transplant centers would benefit from awareness of the efforts required for DAA treatment initiation in the outpatient setting.
To cite this abstract in AMA style:
Berry H, Byrns J, Crona L, Capes T, Steinbrink JM, Maziarz EK, Wolfe CR. Pharmacy Led Initiatives for Treatment of Transplant Recipients of Hepatitis C Viremic Donors in a High Volume Transplant Center [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/pharmacy-led-initiatives-for-treatment-of-transplant-recipients-of-hepatitis-c-viremic-donors-in-a-high-volume-transplant-center/. Accessed February 18, 2026.« Back to 2020 American Transplant Congress