Pharmacokinetic Evaluation of a De Novo Envarsus XR® (tacrolimus Extended Release) Dosing Strategy in Kidney Transplant Recipients
1Department of Pharmacy, Northwestern Memorial Hospital, Chicago, IL, 2Division of Transplantation, Northwestern Memorial Hospital, Chicago, IL
Meeting: 2020 American Transplant Congress
Abstract number: A-091
Keywords: Calcineurin, Immunosuppression, Kidney transplantation
Session Information
Session Name: Poster Session A: Kidney Immunosuppression: Novel Regimens and Drug Minimization
Session Type: Poster Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:00pm
 Presentation Time: 3:30pm-4:00pm
Presentation Time: 3:30pm-4:00pm
Location: Virtual
*Purpose: Envarsus XR® is a once-daily tablet formulation of tacrolimus recently approved for the prophylaxis of organ rejection in de novo kidney transplant recipients (KTx). A phase 2 study of de novo Envarsus XR® utilized a weight-based dosing strategy of 0.14mg/kg/day for non-African American (Non-AA) patients and 0.17mg/kg/day for African American (AA) patients, resulting in supratherapeutic trough concentrations (>11ng/mL) in 11% of recipients after a single dose. This study aims to evaluate an institution-specific initial Envarsus XR® dosing strategy.
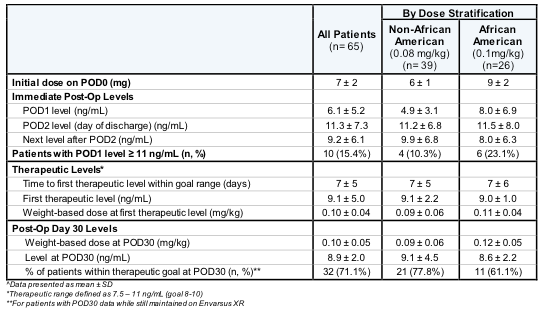
*Methods: This is a single center, retrospective evaluation of adult KTx who received de-novo Envarsus XR® starting POD0 using an institution-specific dosing strategy of 0.08mg/kg/day for Non-AA patients and 0.1mg/kg/day for AA patients (rounded to closest 1mg). Therapeutic goal within 3 months is 8 – 10ng/mL; levels within 7.5 – 11ng/mL were considered within goal based on clinical significance.
*Results: A total of 65 patients were included. Average POD1 level was 6.1 ± 5.2 ng/mL in all patients. POD1 level was <2.0 ng/mL in 18 patients (27.7%), with an average subsequent POD2 level of 7.2 ± 5.4 ng/mL without dose modification. After one dose, 15.4% of all patients had a first level ≥ 11 ng/mL. Average time to therapeutic goal was 7 ± 5 days, with 65.0% of all patients within goal at 7 days post-transplant. At POD30, the weight-based dose was higher than the starting dose in both Non-AA (0.09 ± 0.06 mg/kg) and AA patients (0.12 ± 0.05 mg/kg). A majority of patients were within therapeutic goal at POD30 (71.1%).
*Conclusions: Using the current dosing scheme, a majority of patients had therapeutic tacrolimus levels within 7 days post-op. Weight-based dosing at POD30 was higher than starting doses in both Non-AA and AA patients, but was less than FDA-approved dosing of 0.14 mg/kg/day. As such, the current institution starting dose may need to be slightly increased in both subgroups.
To cite this abstract in AMA style:
Schulte J, Kane C, Cunningham K, D'Agostino C, Kapugi M, Novak A, Shetty A, Leventhal J, Friedewald J. Pharmacokinetic Evaluation of a De Novo Envarsus XR® (tacrolimus Extended Release) Dosing Strategy in Kidney Transplant Recipients [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/pharmacokinetic-evaluation-of-a-de-novo-envarsus-xr-tacrolimus-extended-release-dosing-strategy-in-kidney-transplant-recipients/. Accessed March 1, 2026.« Back to 2020 American Transplant Congress