Phage Therapy for Mycobacterium Abscessus (Mab) Infection after Lung Transplant (LT)
W. Garner1, R. Dedrick2, C. Guerrero2, M. Morrell1, J. D'Cunha3, P. Sanchez1, G. Hatfull2, G. Haidar1
1University of Pittsburgh Medical Center, Pittsburgh, PA, 2University of Pittsburgh, Pittsburgh, PA, 3Mayo Clinic Arizona, Phoenix, AZ
Meeting: 2020 American Transplant Congress
Abstract number: C-190
Keywords: Infection, Lung infection, Lung transplantation
Session Information
Session Name: Poster Session C: All Infections (Excluding Kidney & Viral Hepatitis)
Session Type: Poster Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:00pm
 Presentation Time: 3:30pm-4:00pm
Presentation Time: 3:30pm-4:00pm
Location: Virtual
*Purpose: LT recipients with Mab infections have poor outcomes. We describe a patient with refractory Mab infection treated with mycobacteriophage therapy.
*Methods: A 56-year-old woman with pulmonary fibrosis underwent double LT. 2 months later, she developed a new 9-mm lung nodule. Bronchoalveolar lavage showed many acid-fast bacilli (AFB) and grew Mab subsp. massiliense. Over 4 months, she received combinations of 10 different antibiotics, including clofazimine and bedaquiline. While on therapy, she developed cutaneous Mab nodules, sternal osteomyelitis, and mediastinal abscesses requiring sternotomy and extensive debridement. Cultures continued to grow Mab, prompting us to pursue phage therapy. Using plaque assays, the Mab strain (GD10) was tested for susceptibility to a panel of phages with known activity against M. smegmatis.
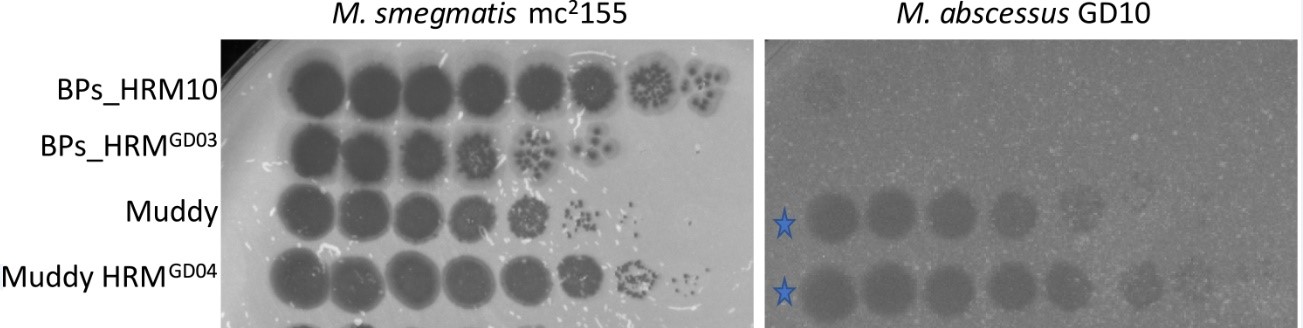
*Results: Most phages did not infect GD10. However, phage Muddy showed complete infection of GD10 with an efficiency of plating of 1 relative to M. smegmatis (Fig1). Due to clinical need, Muddy was used as monotherapy. A high-titer lysate of Muddy was prepared by amplification in M. smegmatis, precipitation, and banding twice in cesium chloride (CsCl) equilibrium density gradient ultracentrifugation. After removal of CsCl by extensive dialysis, the preparation was determined to be sterile with undetectable levels of endotoxin. The final phage titer was 1014 plaque forming units/ml (pfu/ml). An in vitro assay showed that Muddy efficiently kills GD10 over a wide range of conditions. Following FDA eIND and IRB approval, Muddy was administered at 109 pfu IV twice daily, and applied topically and via chest irrigation intra-operatively (dose of 108 pfu). Mab isolates recovered from chest swabs at days 3, 10 and 13 after phage administration remained susceptible to Muddy. She received 28 days of phage with no adverse events. Although most clinical cultures remained positive, her AFB smear burden decreased (heavily smear positive pre-phage; smear-negative or “rare” post-phage). Unfortunately, the patient developed sepsis and expired from unrelated causes.
Fig1: Plaque assay of Muddy, Muddy host range mutant (HRM), and less infective phage (BPs). M. smegmatis = control. G10 = clinical isolate
*Conclusions: IV and topical phage was well-tolerated and reduced the burden of Mab infection, which was progressing despite months of conventional Mab drugs. The role and optimal timing of mycobacteriophage after LT needs to be explored in larger studies.
To cite this abstract in AMA style:
Garner W, Dedrick R, Guerrero C, Morrell M, D'Cunha J, Sanchez P, Hatfull G, Haidar G. Phage Therapy for Mycobacterium Abscessus (Mab) Infection after Lung Transplant (LT) [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/phage-therapy-for-mycobacterium-abscessus-mab-infection-after-lung-transplant-lt/. Accessed February 19, 2026.« Back to 2020 American Transplant Congress