Perturbing Proteostasis to Deplete Plasma Cells
1Cincinnati Children's Hospital, Cincinnati, OH, 2University of Cincinnati College of Medicine, Cincinnati, OH
Meeting: 2022 American Transplant Congress
Abstract number: 282
Keywords: Alloantibodies, B cells, Bone marrow, Highly-sensitized
Topic: Basic Science » Basic Science » 04 - B-cell / Antibody /Autoimmunity
Session Information
Session Name: B-cell / Antibody /Autoimmunity
Session Type: Rapid Fire Oral Abstract
Date: Monday, June 6, 2022
Session Time: 3:30pm-5:00pm
 Presentation Time: 4:00pm-4:10pm
Presentation Time: 4:00pm-4:10pm
Location: Hynes Room 310
*Purpose: Alloantibodies play critical roles in rejection and often prevent sensitized patients from receiving life-sustaining transplants. One approach for enhancing transplantation rates and survival for sensitized patients is based on depleting bone marrow plasma cells (BMPCs), the source of alloantibodies. As part of a clinical trial, we attempted to disrupt BMPC survival by mobilizing them out of the BM with plerixafor and then inhibiting their proteostasis with proteasome inhibitors (PIs).
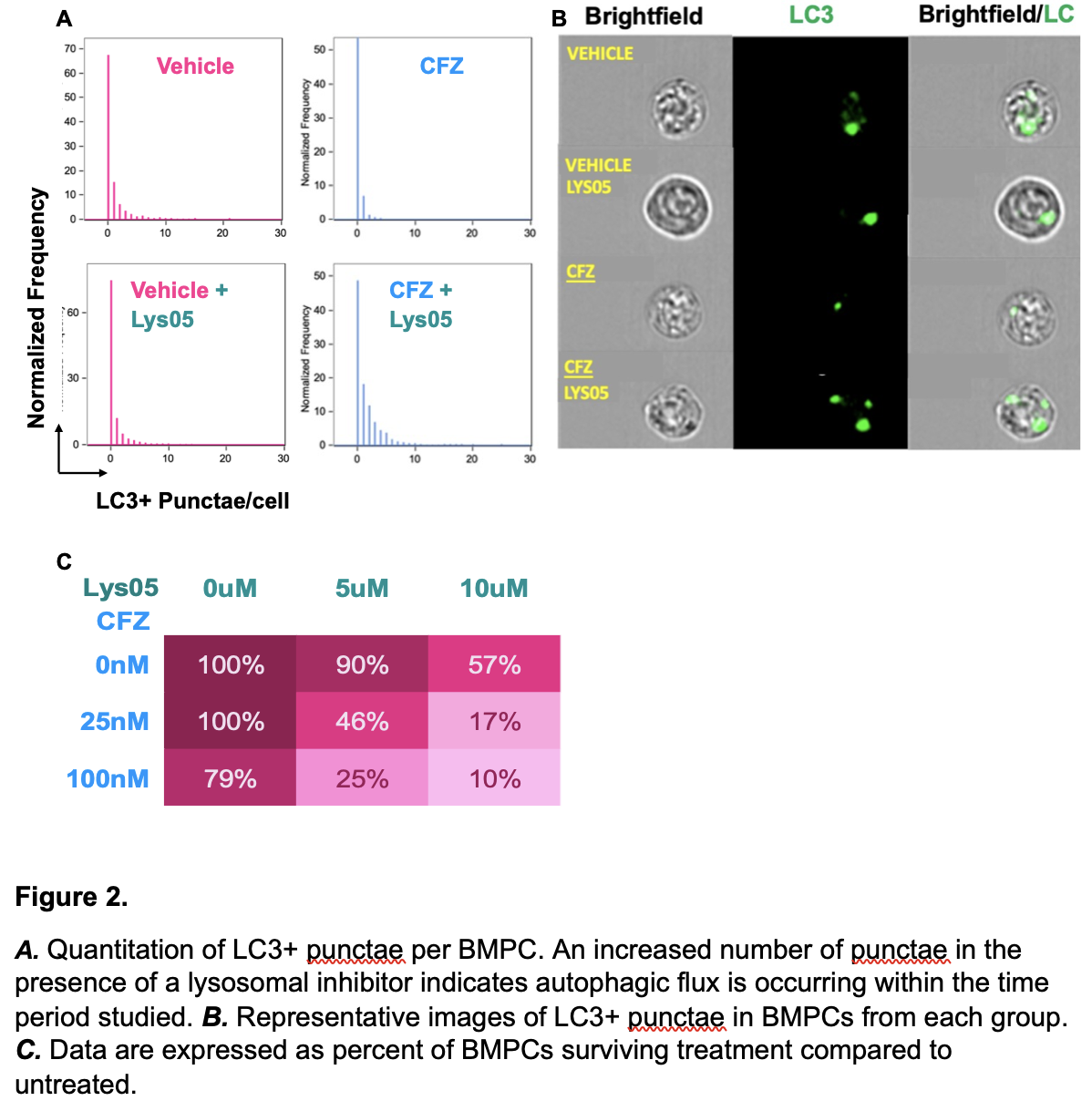
*Methods: Two allosensitized patients (cPRA >20%) enrolled in our trial combining plerixafor and bortezomib to deplete BMPCs (clinicaltrials.gov, NCT02522572) had BM aspirates collected prior to (D-10) and after treatment (D8 or D9). Patients received plerixafor 0.16mg/kg SQ on D0, 1, 2, 3, 5, 6 and bortezomib 1.3 mg/m2 IV on D4, 7. We isolated BMPCs and performed single cell RNA sequencing. Data was processed using CellRanger v6.1 and analyzed using Seurat v4. Autophagic flux was assessed using imaging cytometry to quantitate LC3+ punctae in mouse BMPCs cultured with carfilzomib (CFZ) vs vehicle and the lysosomal inhibitor, Lys05, vs vehicle. Synergy of dually inhibiting the autophagy and proteasome pathways was tested by culturing mouse BMPCs with CFZ or vehicle and Lys05 or vehicle and quantitating BMPC survival by flow cytometry.
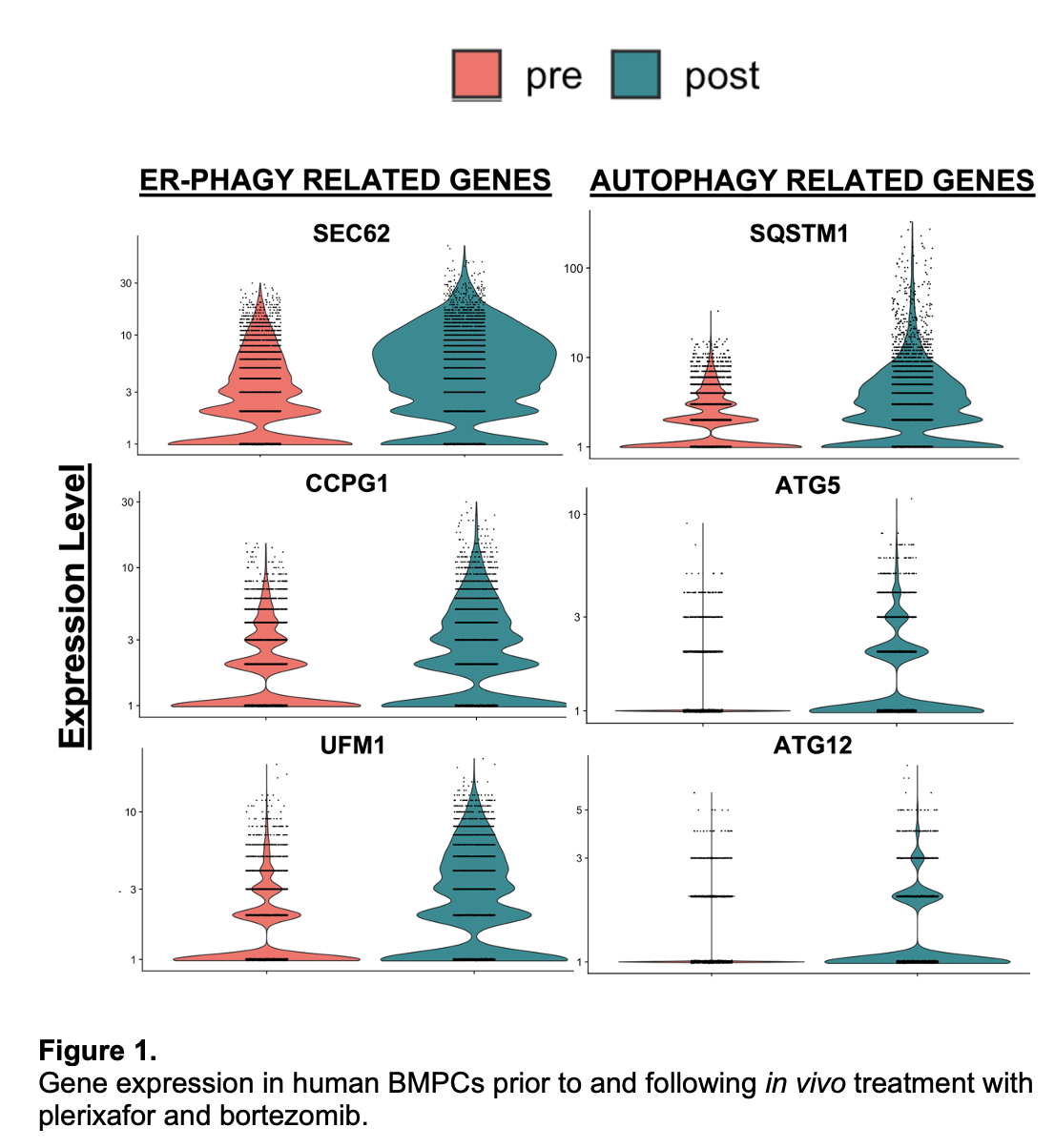
*Results: Human BMPCs surviving plerixafor and PI treatment had increased expression of both general and endoplasmic reticulum autophagy-related genes compared to BMPCs obtained prior to treatment (Fig 1). Specifically, the cargo adaptor protein genes, sqstm1, sec62, and ccpg1 showed robust increases in expression. Mechanistic studies in in vitro murine models demonstrated that proteasome inhibition was sufficient to induce autophagy, as CFZ-treated BMPCs had increased LC3+ punctae and autophagic flux relative to controls (Fig 2A, B). Further, inhibition of autophagy and proteasome pathways synergized to decrease BMPC survival (Fig 2C).
*Conclusions: Data from in vitro murine studies and in vivo human studies demonstrate that BMPCs upregulate autophagy in response to proteasome inhibition and suggest that inhibiting both pathways can enhance BMPC depletion.
To cite this abstract in AMA style:
Rossi A, Burg A, Roskin K, Shields A, Alloway R, Woodle E, Hildeman D. Perturbing Proteostasis to Deplete Plasma Cells [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/perturbing-proteostasis-to-deplete-plasma-cells/. Accessed February 21, 2026.« Back to 2022 American Transplant Congress