Pediatric Primary Kidney Transplant Recipients Outcomes by Immunosuppression Induction Received in the United States
1University of Minnesota, Minneapolis, MN, 2Complex Care Analytics, Fairview Health Services, Minneapolis, MN, 3Surgery, University of Minnesota, Minneapolis, MN, 4Pediatrics, University of Minnesota, Minneapolis, MN
Meeting: 2020 American Transplant Congress
Abstract number: 139
Keywords: Induction therapy, Kidney transplantation, Pediatric, Survival
Session Information
Session Time: 3:15pm-4:45pm
 Presentation Time: 3:27pm-3:39pm
Presentation Time: 3:27pm-3:39pm
Location: Virtual
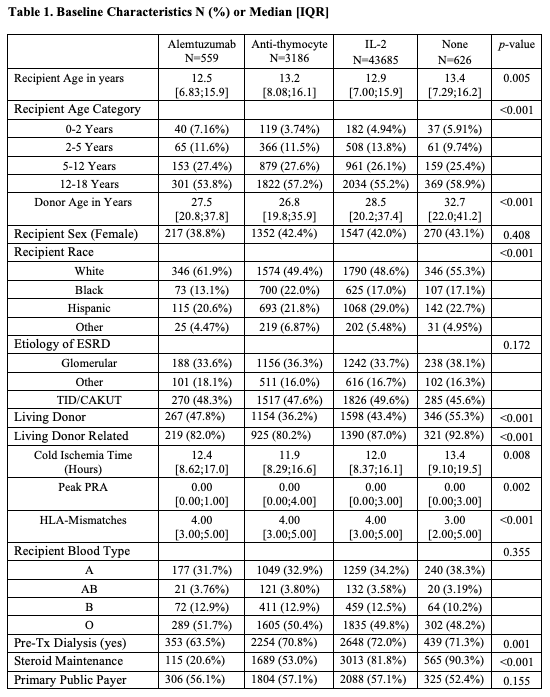
*Purpose: Induction immunosuppression types for pediatric transplant recipients vary widely by transplant centers. We sought to study the impact of different induction types on recipients and graft survival.
*Methods: We analyzed the SRTR standard analysis file to evaluate all primary pediatric kidney transplants receiving tacrolimus and mycophenolate between1995 and 2017. Recipients were grouped by induction type into four groups: Alemtuzumab, Anti-thymocyte, IL-2, and none (steroid only). Kaplan Meier curves were generated for patient and allograft survival by induction type. Predictors for patients and grafts survival were examined using Cox proportional hazards models. The graft survival model was stratified by race and cause of ESRD due to the violation of proportional hazards, and the patient survival stratified by age. Models were adjusted for age, sex, ethnicity, HLA mismatches, PRA, transplant year, cause of ESRD, donor gender, age and type, steroid maintenance and payor type, with transplant center included as a random effect.
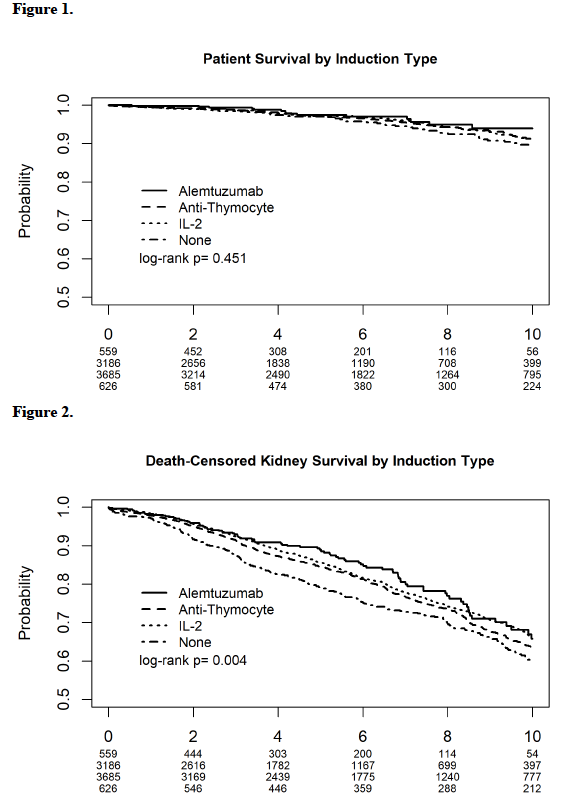
*Results: IL-2 was used in 3685 recipients. Anti-thymocyte was used in 3186 recipients. Alemtuzumab was used in 559 recipients, and 626 recipients received no induction (Table 1). Patient survival was not influenced by induction type (Figure 1). No induction use was associated with the lowest allograft survival log rank 0.004 (Figure 2). Rejection rates at 6 and 12 months did not significantly differ between groups. In the multivariate models, the induction type did not influence patient survival. As compared to no induction, IL-2 was associated with 20% [HR 0.80, 95% C.I. (0.68, 0.95), P 0.011] improved graft survival. Neither alemtuzumab nor anti-thymocyte inductions were associated with benefits.
*Conclusions: In this large cohort of pediatric primary kidney recipients on tacrolimus and mycophenolate maintenance, IL-2 was the only induction regimen with an associated graft survival benefit. IL-2 induction may be the preferred choice in primary pediatric kidney recipients.
To cite this abstract in AMA style:
Riad SM, Jackson S, Matas A, Chinnakotla S, Verghese P. Pediatric Primary Kidney Transplant Recipients Outcomes by Immunosuppression Induction Received in the United States [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/pediatric-primary-kidney-transplant-recipients-outcomes-by-immunosuppression-induction-received-in-the-united-states/. Accessed March 9, 2026.« Back to 2020 American Transplant Congress