Patterns of Belatacept Use and Risk of Post-Transplant Lymphoproliferative Disorder (PTLD) in US Kidney Transplant Recipients – Analysis of Organ Procurement and Transplantation Network (OPTN) Database
W. S. Cherikh1, T. Kou2, J. Foutz1, A. Gomez-Caminero2
1United Network for Organ Sharing (UNOS), Richmond, VA, 2Bristol-Myers Squibb, Princeton, NJ
Meeting: 2020 American Transplant Congress
Abstract number: 500
Keywords: Epstein-Barr virus (EBV), Immunosuppression, Kidney transplantation, Post-transplant lymphoproliferative disorder (PTLD)
Session Information
Session Time: 3:15pm-4:45pm
 Presentation Time: 3:39pm-3:51pm
Presentation Time: 3:39pm-3:51pm
Location: Virtual
*Purpose: Belatacept, a selective T-cell costimulation blocker, was approved in 2011 for the prophylaxis of organ rejection in Epstein-Barr virus (EBV)-seropositive kidney transplant recipients. PTLD is a recognized complication of kidney transplantation and is a safety concern with belatacept use. Here, we report results from two US-based observational studies that utilized data from the OPTN database to examine patterns of belatacept use and the incidence of PTLD in kidney transplant recipients in clinical practice between June 15, 2011 and June 14, 2016.
*Methods: Adult recipients of kidney-alone transplants who received belatacept or calcineurin inhibitor (CNI)-based regimens at transplant were included. Primary objectives were to assess the prevalence of belatacept use, to examine user characteristics including EBV serostatus, and to estimate PTLD incidence rates in adult EBV-seropositive recipients treated with belatacept or CNI at transplant.
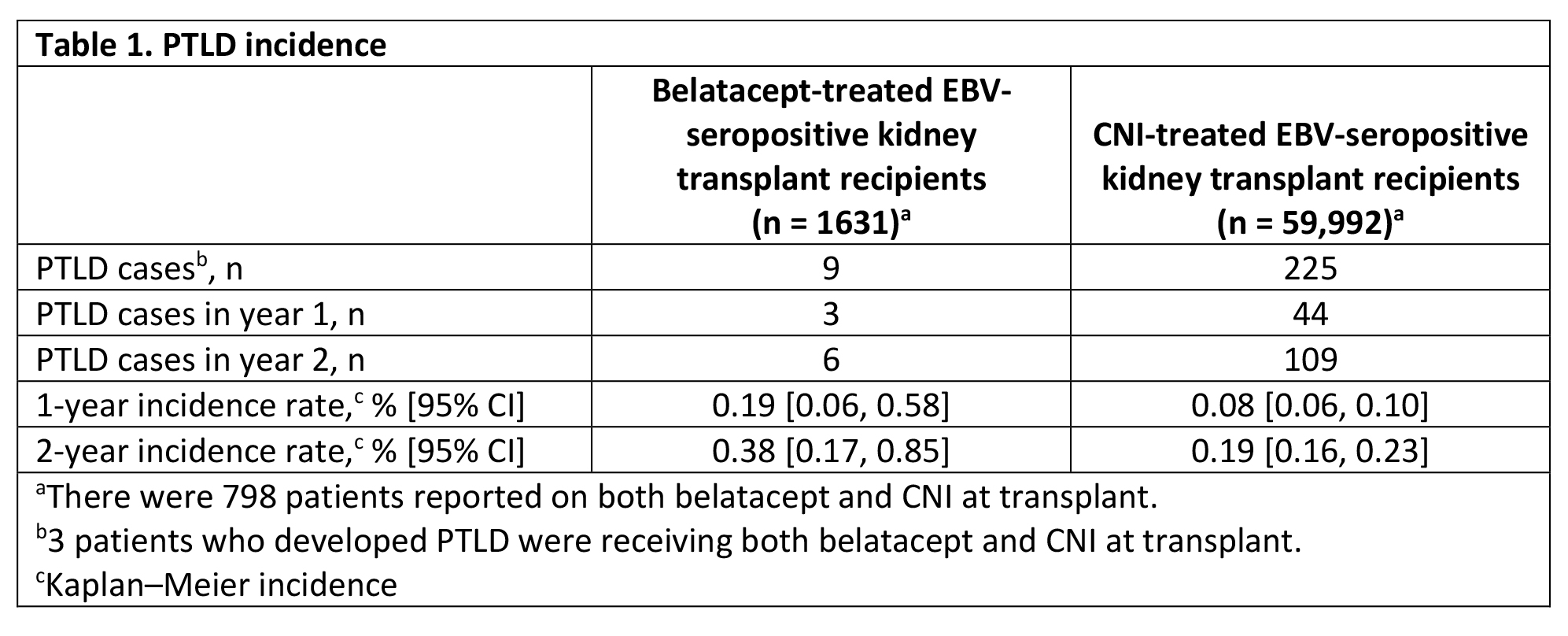
*Results: Overall, 1737 kidney transplant recipients received belatacept at transplant, of whom 1719 had known EBV serostatus and 1631 (93.9% of the overall patient population and 94.9% of patients with known serostatus) were EBV seropositive. A total of 59,992 EBV-seropositive kidney-only transplant recipients received CNI at transplant. In the EBV-seropositive belatacept-treated and CNI-treated groups, respectively, 62.4% and 60.5% were male, 45.1% and 46.6% were aged 40-59 years at transplant, 50.2% and 56.8% received a standard criteria donor kidney, 59.5% and 18.7% received basiliximab for induction, and 22.9% and 50.8% received thymoglobulin for induction. Among EBV-seropositive recipients, 9 PTLD events (2 with known central nervous system [CNS] involvement) were reported in the belatacept group and 225 PTLD events (9 with known CNS involvement) were reported in the CNI group (PTLD incidences are shown in Table 1). PTLD was the cause of death in 4 of 9 belatacept-treated and 81 of 225 CNI-treated patients who developed PTLD. Kaplan-Meier analysis did not show a significant difference in the rates of PTLD between the two groups.
*Conclusions: Since the FDA approval of belatacept in 2011, the majority of adult kidney-only transplant recipients treated with belatacept were EBV seropositive. The risk of PTLD in belatacept-treated EBV-seropositive patients remained low in routine clinical practice and was consistent with observations from previous clinical studies.
To cite this abstract in AMA style:
Cherikh WS, Kou T, Foutz J, Gomez-Caminero A. Patterns of Belatacept Use and Risk of Post-Transplant Lymphoproliferative Disorder (PTLD) in US Kidney Transplant Recipients – Analysis of Organ Procurement and Transplantation Network (OPTN) Database [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/patterns-of-belatacept-use-and-risk-of-post-transplant-lymphoproliferative-disorder-ptld-in-us-kidney-transplant-recipients-analysis-of-organ-procurement-and-transplantation-network-optn-databas/. Accessed March 9, 2026.« Back to 2020 American Transplant Congress