Patient-Reported Outcomes in a Prospective Multicenter Trial of Belatacept-Based CNI- and Corticosteroid-Free Immunosuppression Regimens in Kidney Transplantation: A Prospective Analysis Across Two Years
J. M. Rohan1, J. Leone2, E. Woodle3, D. Kaufman4, A. Shields5, A. Wiseman6, A. Matas7, P. West-Thielke8, E. King5, R. Alloway3
1Pediatrics, Children's Hospital of Richmond at Virginia Commonwealth University, Richmond, VA, 2Tampa General, Tampa, FL, 3University of Cincinnati, Cincinnati, OH, 4U Wisconsin, Madison, WI, 5U Cincinnati, Cincinnati, OH, 6U Colorado, Denver, CO, 7U Minnesota, Minneapolis, MN, 8UIC, Chicago, IL
Meeting: 2020 American Transplant Congress
Abstract number: 220
Keywords: Kidney transplantation, Psychiatric comorbidity, Psychosocial, Quality of life
Session Information
Session Time: 9:59am-10:40am
 Presentation Time: 10:00am-10:07am
Presentation Time: 10:00am-10:07am
Location: Main Channel
*Purpose: Previous trials have not examined 2-year longitudinal differences in patient-reported outcomes (PRO) between belatacept (BELA)- and tacrolimus (TAC)-based therapies in adult kidney transplant (KTx) patients (pts). The BEST Trial (Belatacept-based Early Steroid-withdrawal Trial) was designed to compare 2 BELA-based early steroid withdrawal (ESW) regimens with a TAC-based ESW regimen across 2 years. Our hypothesis was that patients on BELA will have fewer immunosuppressant-related symptoms and improved quality of life (QoL).
*Methods: This longitudinal, multisite study was conducted under an FDA IND and IRB across 8 sites. All pts received mycophenolate therapy and 5 days of steroids. Pts were randomized to 3 groups: alem+BELA, r-ATG+BELA, r-ATG+TAC. Pts completed 2 PRO measures at baseline, 1-year, and 2-years (MEMPHIS, MTSOSD-59R).
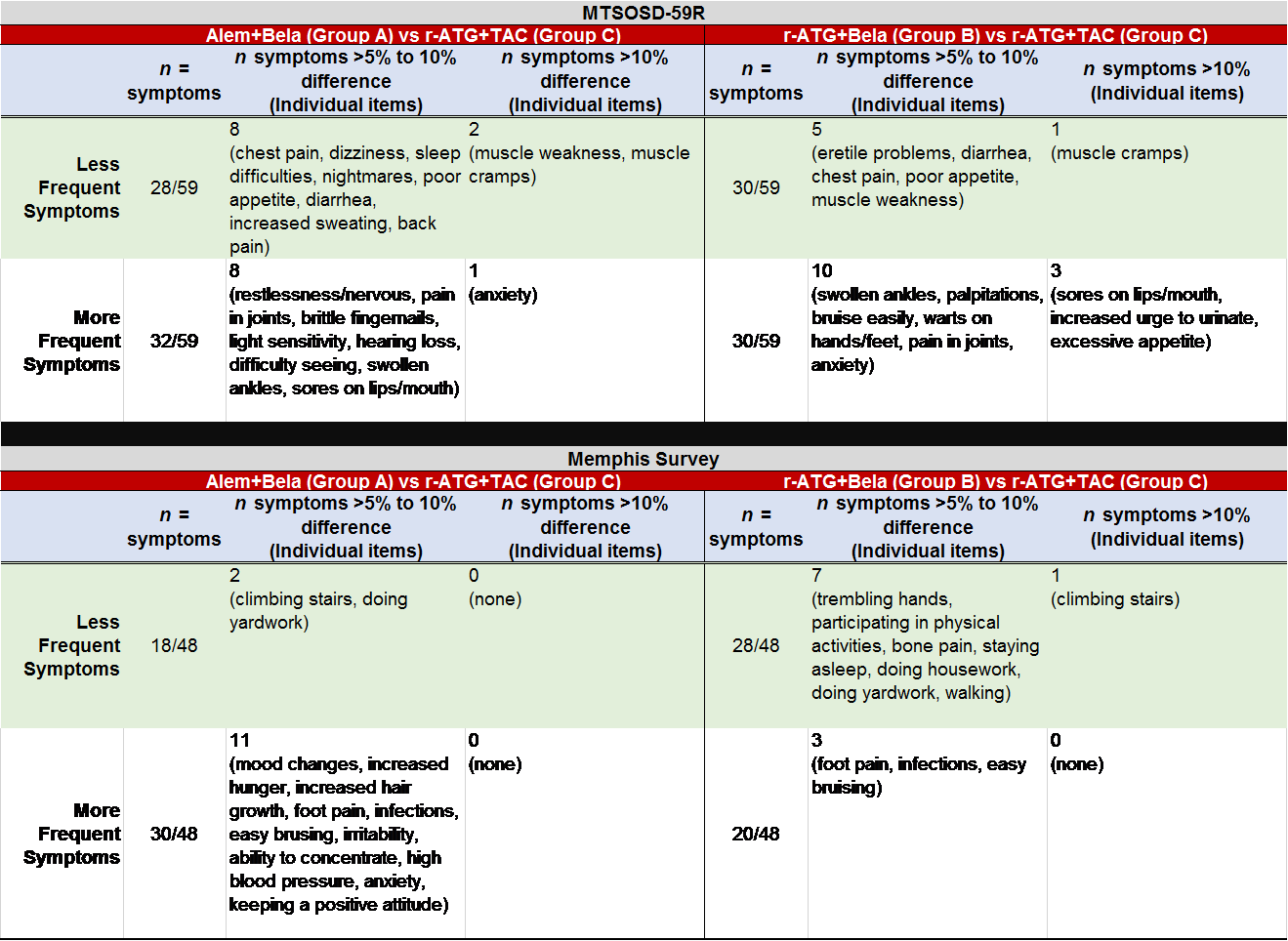
*Results: Intent to treat analyses on 138 pts with 2-year follow-up were completed. Those on BELA-based therapies generally reported higher gains in QoL across 2-years; and, reported lower probability of adverse events at 2-years. No significant differences were observed between groups for total QoL, misc side effects, GI distress, emotional burden, life responsibilities, or mobility. Alem+BELA and r-ATG+BELA groups were compared to r-ATG+TAC group at 2-years and differences in individual symptom scores were observed.
*Conclusions: CNI free/ESW Bela-based regimens resulted in a tendency toward improved PROs across 2-years. All baseline symptoms improved with transplant except GI distress in the TAC-based regimen, which increased at 2-years. Neurologic, GI, and mobility symptoms were reported less frequently in the BELA-based regimens. Psychological symptoms (e.g., restlessness, anxiety, mood changes) were higher in bela-based regimens relative to TAC-based regimens. Sores on lips and warts on hands/feet were more frequently observed in bela-based regimens at 2-years, which may be related to increased viral infections. Bela-based regimens continued to have a decreased risk of trembling hands relative to TAC-based regimens. Table 1. Comparison of Symptom Scores by Treatment Group at 2 years
To cite this abstract in AMA style:
Rohan JM, Leone J, Woodle E, Kaufman D, Shields A, Wiseman A, Matas A, West-Thielke P, King E, Alloway R. Patient-Reported Outcomes in a Prospective Multicenter Trial of Belatacept-Based CNI- and Corticosteroid-Free Immunosuppression Regimens in Kidney Transplantation: A Prospective Analysis Across Two Years [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/patient-reported-outcomes-in-a-prospective-multicenter-trial-of-belatacept-based-cni-and-corticosteroid-free-immunosuppression-regimens-in-kidney-transplantation-a-prospective-analysis-across-two-ye/. Accessed February 23, 2026.« Back to 2020 American Transplant Congress