Patient-Reported Outcomes in a Prospective Multicenter Trial of Belatacept-Based CNI- and Corticosteroid-Free Immunosuppression Regimens in Kidney Transplantation
J. M. Rohan1, J. P. Leone2, E. S. Woodle3, D. Kaufman4, A. R. Shields3, A. Wiseman5, A. J. Matas6, P. West-Thielke7, E. King3, R. R. Alloway3
1Children's Hospital of Richmond at Virginia Commonwealth University, Richmond, VA, 2Tampa General Hospital, Tampa, FL, 3University of Cincinnati College of Medicine, Cincinnati, OH, 4University of Wisconsin – Madison, Madison, WI, 5University of Colorado - Denver, Denver, CO, 6University of Minnesota Medical Center, Minneapolis, MN, 7University of Illinois – Chicago, Chicago, IL
Meeting: 2019 American Transplant Congress
Abstract number: 239
Keywords: Adverse effects, Immunosuppression, Kidney transplantation, Quality of life
Session Information
Session Name: Concurrent Session: Kidney Psychosocial I: Cognitive and Behavioral Factors
Session Type: Concurrent Session
Date: Monday, June 3, 2019
Session Time: 2:30pm-4:00pm
 Presentation Time: 2:30pm-2:42pm
Presentation Time: 2:30pm-2:42pm
Location: Room 302
*Purpose: Previous trials have not examined differences in patient-reported outcomes (PRO) between belatacept (BELA)- and tacrolimus (TAC)-based therapies in adult kidney transplant (KTx) patients (pts). The BEST Trial (Belatacept-based Early Steroid-withdrawal Trial) was designed to compare 2 BELA-based early steroid withdrawal (ESW) regimens with a TAC-based ESW regimen across 2 years. Our hypothesis was that patients on BELA will have fewer immunosuppressant-related symptoms and improved quality of life (QoL).
*Methods: This longitudinal, multisite study was conducted under an FDA IND and IRB across 8 sites. All pts (n=315) received mycophenolate therapy and 5 days of steroids; and, were randomized to 3 groups: alemtuzumab+BELA, r-ATG+BELA, and r-ATG+TAC. Pts completed two PRO measures at baseline and 12 months (MEMPHIS Survey, MTSOSD-59R Survey).
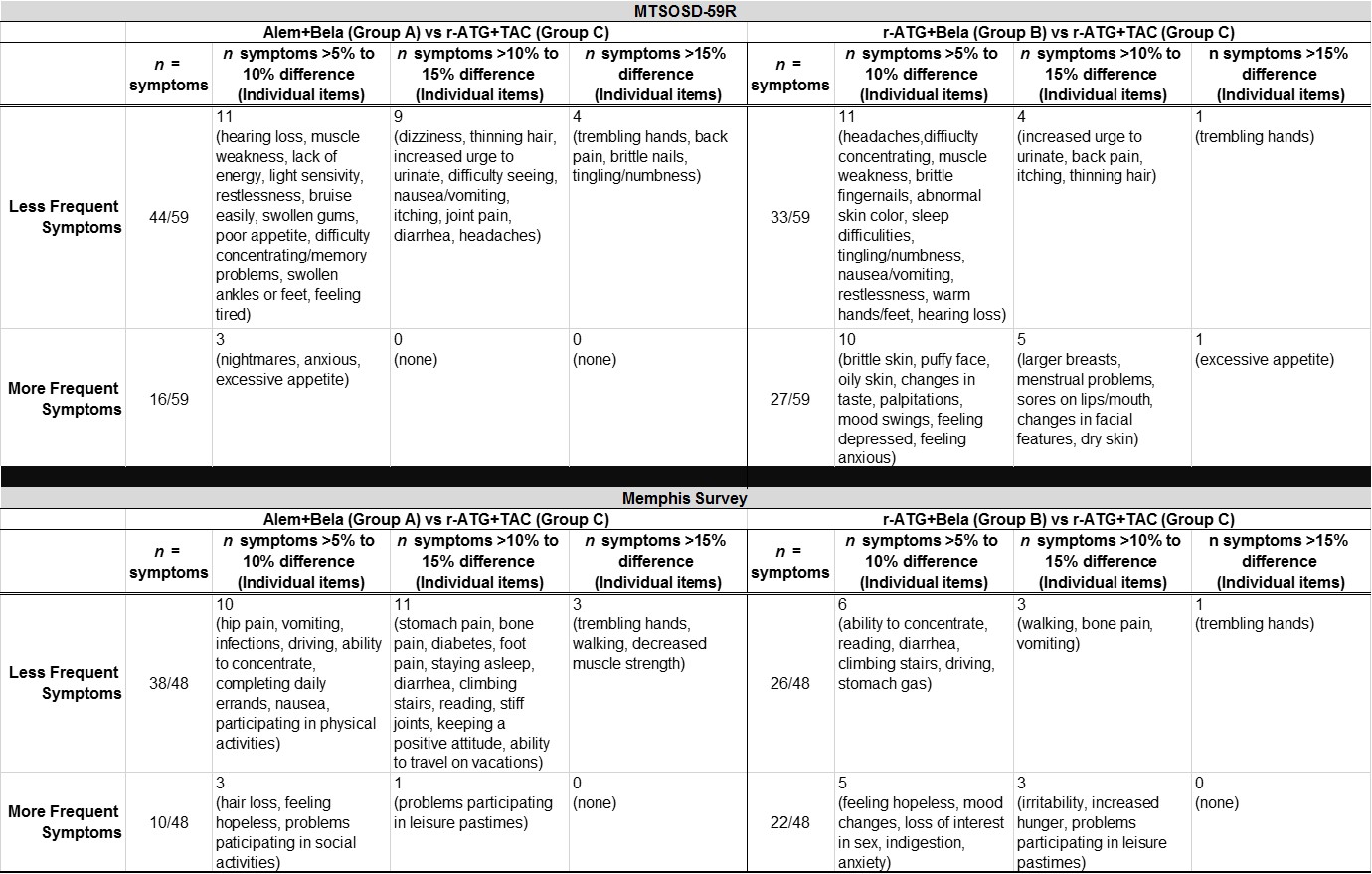
*Results: Intent to treat analyses on 264 patients with 1 year follow-up were completed. Those on BELA-based therapies generally reported higher gains in quality of life from baseline to one year; and, reported a lower probability of experiencing adverse events at one year. No significant differences were observed between groups for total QoL, misc side effects, GI distress, emotional burden, life/role responsibilities or mobility as measured by the MEMPHIS change weighted scores or the symptom occurrence or symptom distress scales in the MTSOSD-59R. Alemtuzumab+BELA and r-ATG+BELA groups were compared to r-ATG+TAC group at 1 year and differences in individual symptom scores were observed.
Table 1. Comparison of Symptom Scores by Treatment Group at 1 year
*Conclusions: Our results show that a TAC free and steroid free BELA-based regimen resulted in a tendency toward improved PROs at 1 year. Neurologic, gastrointestinal, and mobility symptoms were reported less frequently in the BELA-based regimens across both measures with a >15% difference reported in trembling hands. BELA-based protocols reported more problems participating in leisure past-times.
To cite this abstract in AMA style:
Rohan JM, Leone JP, Woodle ES, Kaufman D, Shields AR, Wiseman A, Matas AJ, West-Thielke P, King E, Alloway RR. Patient-Reported Outcomes in a Prospective Multicenter Trial of Belatacept-Based CNI- and Corticosteroid-Free Immunosuppression Regimens in Kidney Transplantation [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/patient-reported-outcomes-in-a-prospective-multicenter-trial-of-belatacept-based-cni-and-corticosteroid-free-immunosuppression-regimens-in-kidney-transplantation/. Accessed February 19, 2026.« Back to 2019 American Transplant Congress