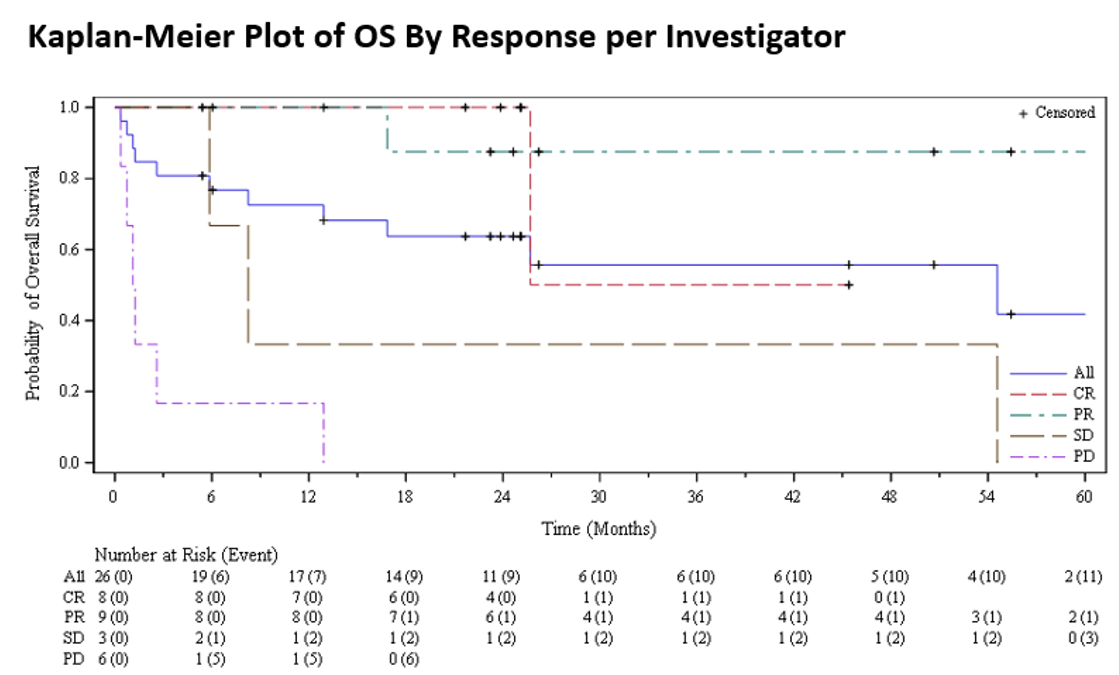
Overall Survival by Best Overall Response with Tabelecleucel in Patients with Epstein-Barr Virus-Driven Post-Transplant Lymphoproliferative Disease After Solid Organ Transplant
S. Prockop1, L. Gamelin2, R. Dinavahi2, Y. Sun2, N. Guzman-Becerra2, H. Parmar2
1Memorial Sloan Kettering Cancer Center, New York, NY, 2Atara Biotherapeutics, South San Francisco, CA
Meeting: 2021 American Transplant Congress
Abstract number: 197
Keywords: Epstein-Barr virus (EBV), High-risk, Post-transplant lymphoproliferative disorder (PTLD), Survival
Topic: Clinical Science » Infectious Disease » PTLD: All Topics
Session Information
Session Time: 10:30am-11:30am
 Presentation Time: 10:30am-10:40am
Presentation Time: 10:30am-10:40am
Location: Virtual
*Purpose: Tabelecleucel is an investigational, off-the-shelf, allogeneic Epstein-Barr virus (EBV)-specific T-cell immunotherapy being studied in patients (pts) with serious EBV-driven diseases, including post-transplant lymphoproliferative disease (EBV+ PTLD). Pts with EBV+ PTLD after solid organ transplant (SOT) who relapsed with rituximab and did not respond to or did not receive additional chemotherapy (CT) had a median overall survival (OS) of <3 months (Zimmermann EHA 2019), demonstrating a substantial unmet need in relapsed/refractory (R/R) EBV+ PTLD after SOT. We have previously shown that pts with EBV+ PTLD after SOT who responded (complete response [CR] or partial response [PR]) to tabelecleucel have clinical benefit, including 100% 2-year survival rates (Prockop ASH 2019 and JCI 2019). Here, we report aggregate OS in patients with EBV+ PTLD after SOT with CR or PR with tabelecleucel treatment.
*Methods: Treatment response and OS were assessed in three studies (NCT00002663, NCT01498484 and NCT02822495). All pts received tabelecleucel at ≈2 x 106 cells/kg on Days 1, 8 and 15 in a 35-day treatment cycle. Pts received a median (range) of 2 (1-9) cycles.
*Results: Twenty-six SOT recipients with EBV+ PTLD R/R to rituximab (SOT1 n=7) or rituximab+CT (SOT2 n=19) were treated. The objective response rate (PR+CR) was 65% (17/26) overall, 86% (6/7) in SOT1, and 58% (11/19) in SOT2. Similar survival rates were observed across pts with best overall response (BOR) of CR or PR, including in the SOT1 and SOT2 subgroups (Table 1, Figure 1). Treatment was well tolerated with no confirmed evidence for graft vs host disease, cytokine release syndrome or neurotoxicity in relation to tabelecleucel in these very sick, treatment refractory, and immunocompromised pts.
*Conclusions: These data show that not only pts with complete responses, but also pts with partial responses to tabelecleucel, may obtain longer-term clinical benefit, demonstrating a favorable risk‒benefit profile in this high-risk population for whom there are no approved alternative therapies.
| All SOT EBV+ PTLD (n=26) | SOT1 (n=7) |
SOT2 (n=19) |
||||
| BOR | CR (n=8) | PR (n=9) | CR (n=4) | PR (n=2) | CR (n=4) | PR (n=7) |
| 1-year OS rate | 100% | 100% | 100% | 100% | 100% | 100% |
| 2-year OS rate (95% CI) |
100% | 87.5% (38.7,98.1) |
100% | 100% | 100% | 83.3% (27.3,97.5) |
| Median follow-up (min,max) months |
24.5 (6.0,45.4) |
26.2 (5.4,115.0) |
22.8 (12.9,25.7) |
38.4 (26.2,50.7) |
25.1 (6.0,45.4) |
24.6 (5.4,115.0) |
To cite this abstract in AMA style:
Prockop S, Gamelin L, Dinavahi R, Sun Y, Guzman-Becerra N, Parmar H. Overall Survival by Best Overall Response with Tabelecleucel in Patients with Epstein-Barr Virus-Driven Post-Transplant Lymphoproliferative Disease After Solid Organ Transplant [abstract]. Am J Transplant. 2021; 21 (suppl 3). https://atcmeetingabstracts.com/abstract/overall-survival-by-best-overall-response-with-tabelecleucel-in-patients-with-epstein-barr-virus-driven-post-transplant-lymphoproliferative-disease-after-solid-organ-transplant/. Accessed March 9, 2026.« Back to 2021 American Transplant Congress