Outpatient Treatment of Paramyxovirus with Oral Ribavirin is Safe and Effective in Lung Transplant Recipients
1Department of Pharmacy, Houston Methodist Hospital, Houston, TX, 2Department of Medicine, Houston Methodist Hospital, Houston, TX
Meeting: 2020 American Transplant Congress
Abstract number: B-288
Keywords: Infection, Lung transplantation
Session Information
Session Name: Poster Session B: Lung: All Topics
Session Type: Poster Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:00pm
 Presentation Time: 3:30pm-4:00pm
Presentation Time: 3:30pm-4:00pm
Location: Virtual
*Purpose: Respiratory paramyxovirus infections present an increased risk to lung transplant recipients (LTRs), including rapid decline in graft function and immunologic sequelae such as rejection or donor specific antibody (DSA) formation following infection. Inhaled ribavirin (RBV) has been a mainstay of treatment, but imposes a significant logistical and cost burden with a 5 day course for medication costing $138,000 (AWP cost). Alternatively oral RBV is readily available and less expensive, but it presents significant safety concerns with anemia in an already myelosuppressed population. In 2018 our center began utilizing outpatient treatment of paramyxovirus in LTRs with a 10 day course of oral RBV at 400 mg TID ($496 AWP cost). Herein we report our initial experience with oral RBV therapy for paramyxovirus infections in the outpatient setting.
*Methods: We performed a single center, retrospective review of all positive respiratory pathogen panels (Biofire® FilmArray® Respiratory Panel) from nasopharyngeal swab or bronchial wash from 10/1/2018 through 9/30/2019. A total of 551 PCR assays were positive for respiratory syncytial virus (RSV) or human metapneumovirus (hMPV), with 79 occurrences in LTRs. Of these, 16 received outpatient oral RBV, and were included for safety and efficacy analyses.
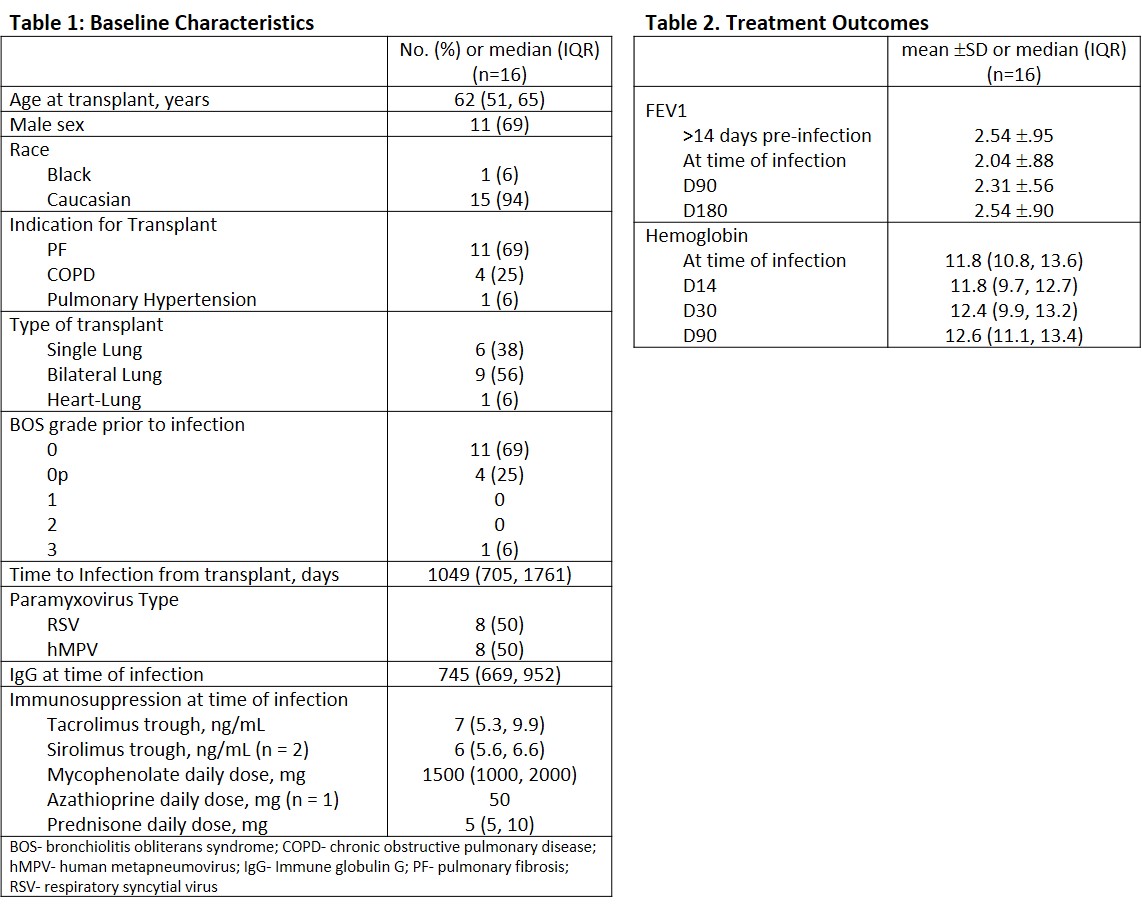
*Results: Infections were seen predominately in older males with BOS grade 0 and occurred at a median of 1,049 days post-transplant (IQR). The majority of patients received bilateral lung transplants for pulmonary fibrosis. Baseline characteristics are described in Table 1. Two patients had concomitant viral respiratory infections, coronavirus and parainfluenza. No concomitant bacterial infections were identified. All patients were treated with 10 days of oral RBV and prednisone taper. One patient received IVIG. There were no outpatient treatment failures. Median BOS prior to infection was grade 0, 3 patients had BOS progression by 3 months post infection. FEV1 change from time of infection through 6 months follow up is described in Table 2. One patient developed DSA post infection, but no acute rejection episodes were observed. No significant change in CBC was seen during treatment with RBV, or out to 6 months post.
*Conclusions: Outpatient administration of oral RBV may be safe and effective for treatment of paramyxovirus in lung transplant while ameliorating significant medication costs.
To cite this abstract in AMA style:
Rogers AW, Pierce BJ, Pham C, Swan JT, Huang HJ. Outpatient Treatment of Paramyxovirus with Oral Ribavirin is Safe and Effective in Lung Transplant Recipients [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/outpatient-treatment-of-paramyxovirus-with-oral-ribavirin-is-safe-and-effective-in-lung-transplant-recipients/. Accessed March 2, 2026.« Back to 2020 American Transplant Congress