Outcomes of Intra-Operative Steroid Induction in Liver Transplant Recipients
Henry Ford Hospital, Detroit, MI
Meeting: 2020 American Transplant Congress
Abstract number: B-136
Keywords: Immunosuppression, Induction therapy
Session Information
Session Name: Poster Session B: Liver: Immunosuppression and Rejection
Session Type: Poster Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:00pm
 Presentation Time: 3:30pm-4:00pm
Presentation Time: 3:30pm-4:00pm
Location: Virtual
*Purpose: Evidence supporting the use of antibody induction (AI) immunosuppression (IS) (antithymocyteglobulin (rATG) and basiliximab (BAS)) for acute rejection (AR) prophylaxis in liver transplant is controversial, and evidence for its use is not as conclusive as it is in other organs. The purpose of this study is to evaluate outcomes after induction protocol change from AI to steroids alone.
*Methods: This is a retrospective quasi experimental single-center descriptive study evaluating incidence of biopsy proven acute rejection (BPAR) between patients (pts) who received AI with rATG or BAS compared to those who did not. Primary outcome assessed the incidence of BPAR, and secondary outcomes assessed the incidence of infection, malignancy, and graft versus host disease (GVHD) up to a year post transplant.
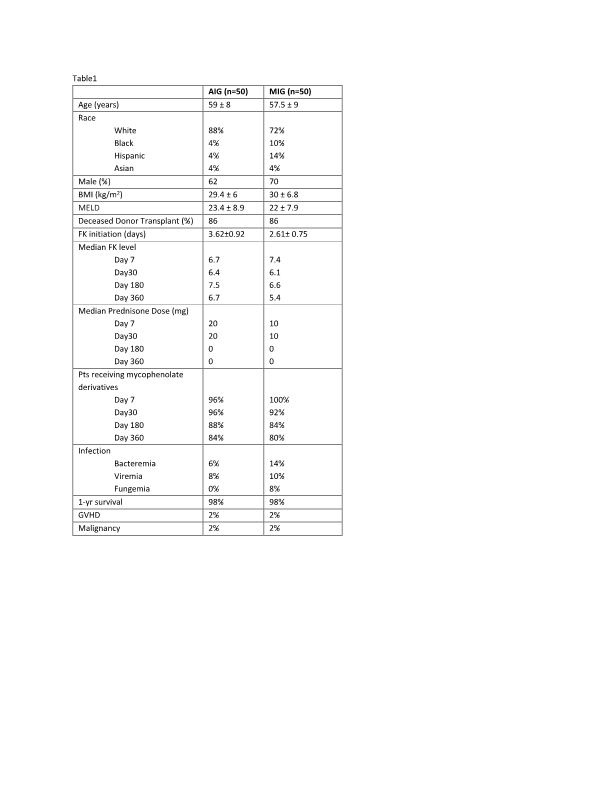
*Results: 100 pts were included, 50 in the AI group (AIG) (74% rATG, 26% BAS) and 50 in the methylprednisolone induction group (MIG), with baseline characteristics shown in Table1. 98 pts received triple maintenance IS of tacrolimus (FK), mycophenolate derivatives, and prednisone within 7 days of transplant; levels/doses similar at day 30, 180, and 360 (Table1). In the AIG, 10 (20%) pts experienced ≥1 BPAR: 9 acute cellular rejection (ACR) and 1 antibody mediated rejection (AMR). In the MIG, 19(38%) pts experienced ≥1 BPAR: 24 ACR and 1 AMR. Median time to rejection was 135 days (85-185) in AIG vs. 103 days (58-161) in the MIG. Pts in the MIG, received an average of 2350mg ±1289 of prednisone equivalents vs. 1835mg ± 921 in the AIG, and had higher incidence of infections, with no change in other negative outcomes.
*Conclusions: Use of steroids alone resulted in more BPAR, shorter time to rejection, and higher rates of infections compared to antibody induction.
To cite this abstract in AMA style:
Poparad-Stezar A, Jantz A, Larson T, Summers B, Sulejmani N. Outcomes of Intra-Operative Steroid Induction in Liver Transplant Recipients [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/outcomes-of-intra-operative-steroid-induction-in-liver-transplant-recipients/. Accessed March 8, 2026.« Back to 2020 American Transplant Congress